Chapter 16 Chemistry of Benzene 47 A. Electrophillic aromatic
advertisement

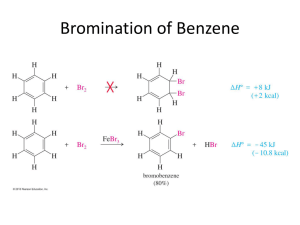
Chapter 16 Chemistry of Benzene A. Electrophillic aromatic substitution of benzene rings 1. Monosubstitution - If compound does electrophilic substitution rather than addition – it is most likely aromatic. - some possibilities Addition reaction Substitution reaction - less reactive benzene ring requires a Lewis acid catalyst to promote reaction by making the electrophile more electrophilic. doubly allylic carbocation – three resonance structures 47 Chapter 16 Chemistry of Benzene Reaction of benzene is slower than alkenes because benzene is more stable. Both reactions are endergonic to make carbocation intermediate. • • Benzene has a larger Ea. Subtitituion of H not addition at carbocation get back aromatic stable structure. If forms the addition product lose aromatic stability. Endergonic reaction is then observed. This overall is an exergonic reaction. 48 Chapter 16 Chemistry of Benzene i) Aromatic bromination: Mechanism. 16.2 Other aromatic substitutions: These occur by a similar mechanism. ii) Halogens (Cl2, I2) - F2 is too reactive and give poor yields – Anti-anxiety drug – Valium. 49 Chapter 16 Chemistry of Benzene First oxidize Iodine with Cu( II) salt or hydrogen peroxide to give more electrophilic I⊕ iii) Aromatic Nitration: The catalyst is a mixture of Conc. H2SO4 & conc. HNO3 to give NO2⊕ Sulfuric acid protonates the HNO3, subsequent loss of water gives the Nitronium ion Nitronium ion is very electrophilic – deprotonation of the Hydrogen regenerates the catalyst and forms the substituted product. • • reduction of Nitrobenzene use Fe, Sn or SnCl2 Aniline for dyes and pharmaceuticals More in chapter 24. 50 Chapter 16 Chemistry of Benzene iv) Aromatic sulfonation – making benzenesulfonic acid. Catalyst – Fuming sulphuric acid (mixture of H2SO4 and SO3) Electrophile is HSO3⊕ or neutral SO3 Sulfonation product for dyes & pharmaceuticals This reaction is reversible. Sulfonation product favoured by strong acid… Reverse favored by hot, dilute aqueous acid. Aromatic sulfonation is a key step towards making Sulfanilamide – an antibiotic - now it is used to treat meningitis and urinary tract infections. 51 Chapter 16 Chemistry of Benzene 16.3 Alkylation and Acylation of Aromatic rings v) Friedel-Craft alkylation - Need alkyl chloride and Lewis acid catalyst AlCl3 Electrophile is an alkyl carbocation. Cl AlCl3 AlCl4 H AlCl4 AlCl4 + HCl + AlCl3 Limitations of Friedal-Craft reactions 1. No reaction with aryl halide or vinylic halide – carbocations are too high energy. Cl Cl 2. No reaction - making disubstituted rings by Friedal-Craft when strongly electron withdrawing group is present. Presence of Amino group, or C=O (carbonyl group) 52 Chapter 16 Chemistry of Benzene 3. Difficult to stop with a single substitution. – Poly alkylation is often observed. - high yield of monosubtitution product is obtained by using large excess of benzene. 4. Rearrangement of alkyl carbocation when primary alkyl halide is used. 1,2-H-shift to form a more stable carbocation 1-chlorobutane 1,2-methyl shift. Neopentyl chloride (1-chloro-2,2-dimethylpropane) 53 Chapter 16 Chemistry of Benzene vi) Friedal-Craft acylation No carbocation rearrangement - acyl carbocation is resonance stabilized. Acyl cation is stabilized by donation of electrons from lone pairs on O to Carbocation's vacant – p-orbital. Only monosubstitution – no polysubstitution. - C=O – acylated benzene is less reactive. Do questions 16.4, 16.5, 16.6 54 Chapter 16 Chemistry of Benzene 2. Disubstitution 16.4 Substituent effects in substituted aromatics rings. Presence of a substituent has two kinds of effects on the ring A) Reactivity of the ring i) Activate – make benzene ring more reactive ii) Deactivate – make benzene ring less reactive. B) Orientation of new substituent: ortho, meta, para. • • The formation of these disubstituted products are not equal. Nature of the substituent already present will determine the position of second. Classification of the substituent already present into three categories: 1. 2. 3. Ortho and para directing activators Ortho and para directing deactivators Meta directing deactivators Note: There are no meta directing deactivators Directing effect correlate to reactivities. 1. 2. 3. All meta directing groups are deactivators Most ortho- and para- directing groups are activators Halogens are weak ortho- and para- directing deactivators. 55 Chapter 16 Chemistry of Benzene Reactivity and orientation: Two key factors - Inductive effect and resonance effect. Inductive effect: Donation or withrdawl of electrons through σ-bond due to electronegativity. Donating groups: Alkyl groups Withdrawing groups: Halogens, Cyano (-C≡N), hydroxyl, Carbonyl groups, Nitro Halogen and Hydroxyl – electronegative atom directly attached. Rest are -Y-Z type (where Y is attached to ring and Z is electronegative atom). 56 Chapter 16 Chemistry of Benzene Resonance effect: Withdrawal or donation of electrons through π-bond due to p-orbital overlap on the subsitutent with p-orbital on aromatic ring. Withdrawing groups: Carbonyl, Cyano, Nitro – have Y-Z , where Z>Y in electronegativity These leave a positive charge on the ring. Donating groups: Halogen, Hydroxyl, alkoxy, amino Note: these have at least a lone pair of electrons – these electrons can be pushed into the ring to give the ring a negative charge. Note: inductive effect and resonance effect counteract each other for this type of substituent.; the stronger effect will dominate.`` 57 Chapter 16 Chemistry of Benzene 16.5 Explanation of substituent effects: Common characteristics Activating: electrons donate to ring. This stabilizes carbocation intermediate and lower activatioin energy for its formation. -OH, -alkoxy, amino groups – donate π electrons (resonance effect) Stronger than EW. - alkyl groups donate through inductive effect. Deactivating: electrons are withdrawn from the ring. This destabilizes the carbocation intermediate and raises the energy for its formation. Carbonyl, cyano, nitro groups are examples. Have both inductive effect and resonance effect to withdraw electrons from the ring Halogens are deactivating – strong EW (σ- inductive) and weak ED (π-resonance) There are three possible types of activators and deactivators. 1) Ortho & Para – directing activators - alkyl groups, OH, and NH2 2) Ortho & Para – directing deactivators – Halogens 3) Meta – directing deactivators - Carbonyl, CN, NO2 58 Chapter 16 Chemistry of Benzene Ortho and Para- directing activators – Alkyl groups. Electron donating inductive effect – no resonance effect. Nitration of toluene- can occur in the ortho, meta, or para positions. In each case, the most stable carbocation intermediate is obtained when substitution occurs at the ortho or para positions. - Tertiary carbocation is more stable than secondary. 59 Chapter 16 Chemistry of Benzene Ortho and para –directing activators - OH and NH2 groups. Stronger ED π, Weaker EW σ Stabilization of the carbocation by resonance effect – electron donation of Oxygen's lone pair to positively charged carbon. – only ortho and para position have this effect. Ortho and para – directing deactivators: Halogens - Stronger EW σ, and weaker ED π. Electron donating π is weak but felt more at ortho and para positions. Stabilize carbocation intermediate when 2nd substituent is placed in ortho or para positions. Same diagram as above – relative amounts: ortho 35%, para 64% This is considered deactivating because the EW is stronger than ED. Activating requires Stronger ED. 60 Chapter 16 Chemistry of Benzene Meta directing deactivators: Have stronger EW (both σ& π) effect is felt stronger at ortho and para – destabilizes it. so that meta substitution occurs. Charge on the ipso carbon is not good especially with electron withdrawing group next to it. This table is a nice summary of different effects on aromatic substitution. 61 Chapter 16 Chemistry of Benzene 16.6 Trisubstituted benzene Resonance and inductive effects still applies. Now, determine additive effect of two groups. 1. Reinforcement of two groups – direct to same position 2. Contradictory – more powerful activating group wins. 3. Steric hindrance between meta substituted group – no substitution 62 Chapter 16 Chemistry of Benzene Do suggested questions in textbook 6th edition: 16.1, 16.4, 16.5, 16.6, 16.7, 16.8, 16.9, 16.10, 16.12, 16.13, 16.14, 16.15, 16.28, 16.29, 16.30, 16.31, 16.32, 16.33, 16.34, 16.35, 16.36, 16.42, 16.51, 16.52, 16.53, 16.64 7th edition: 16.1, 16.4, 16.5, 16.6, 16.7, 16.8, 16.9, 16.10, 16.12, 16.13, 16.14, 16.15, 16.29, 16.30, 16.31, 16.32, 16.33, 16.34, 16.35, 16.36, 16.37, 16.43, 16.52, 16.53, 16.54, 16.65 63