Water Properties Lab
advertisement
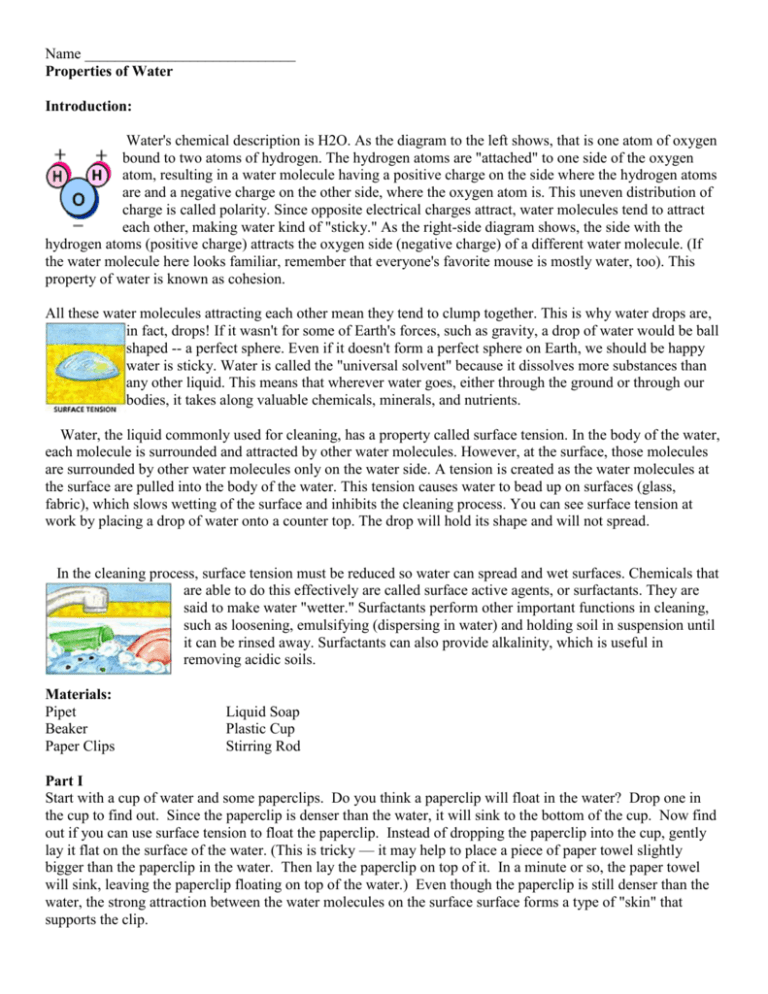
Name ____________________________ Properties of Water Introduction: Water's chemical description is H2O. As the diagram to the left shows, that is one atom of oxygen bound to two atoms of hydrogen. The hydrogen atoms are "attached" to one side of the oxygen atom, resulting in a water molecule having a positive charge on the side where the hydrogen atoms are and a negative charge on the other side, where the oxygen atom is. This uneven distribution of charge is called polarity. Since opposite electrical charges attract, water molecules tend to attract each other, making water kind of "sticky." As the right-side diagram shows, the side with the hydrogen atoms (positive charge) attracts the oxygen side (negative charge) of a different water molecule. (If the water molecule here looks familiar, remember that everyone's favorite mouse is mostly water, too). This property of water is known as cohesion. All these water molecules attracting each other mean they tend to clump together. This is why water drops are, in fact, drops! If it wasn't for some of Earth's forces, such as gravity, a drop of water would be ball shaped -- a perfect sphere. Even if it doesn't form a perfect sphere on Earth, we should be happy water is sticky. Water is called the "universal solvent" because it dissolves more substances than any other liquid. This means that wherever water goes, either through the ground or through our bodies, it takes along valuable chemicals, minerals, and nutrients. Water, the liquid commonly used for cleaning, has a property called surface tension. In the body of the water, each molecule is surrounded and attracted by other water molecules. However, at the surface, those molecules are surrounded by other water molecules only on the water side. A tension is created as the water molecules at the surface are pulled into the body of the water. This tension causes water to bead up on surfaces (glass, fabric), which slows wetting of the surface and inhibits the cleaning process. You can see surface tension at work by placing a drop of water onto a counter top. The drop will hold its shape and will not spread. In the cleaning process, surface tension must be reduced so water can spread and wet surfaces. Chemicals that are able to do this effectively are called surface active agents, or surfactants. They are said to make water "wetter." Surfactants perform other important functions in cleaning, such as loosening, emulsifying (dispersing in water) and holding soil in suspension until it can be rinsed away. Surfactants can also provide alkalinity, which is useful in removing acidic soils. Materials: Pipet Beaker Paper Clips Liquid Soap Plastic Cup Stirring Rod Part I Start with a cup of water and some paperclips. Do you think a paperclip will float in the water? Drop one in the cup to find out. Since the paperclip is denser than the water, it will sink to the bottom of the cup. Now find out if you can use surface tension to float the paperclip. Instead of dropping the paperclip into the cup, gently lay it flat on the surface of the water. (This is tricky — it may help to place a piece of paper towel slightly bigger than the paperclip in the water. Then lay the paperclip on top of it. In a minute or so, the paper towel will sink, leaving the paperclip floating on top of the water.) Even though the paperclip is still denser than the water, the strong attraction between the water molecules on the surface surface forms a type of "skin" that supports the clip. Part II Cohesiveness of Water: 1. Estimate how many paper clips will fit into a completely full cup of water. Record this number in data table below. 2. Fill a plastic cup with tap water. 3. Pour tap water from your cup into your beaker 4. Continue to add water by pipet until the top surface appears rounded. 5. Slowly add paper clips one at a time to the beaker keeping count of all paper clips that you add. 6. Stop adding paper clips to the beaker whenever water spills from the top. 7. Record your paper clip count. Compare the actual number of paper clips to the estimated number. Table 1 Cohesiveness of Tapwater Estimated Number of Paper Actual Number of paper Clips Clips Difference Part III Procedure (Part B) Soap's effect on Surface Tension: 1. Again estimate how many paper clips will fit into a completely full beaker of soapy water. Record this number in data table 2. Fill a plastic cup with tap water. 3. Add 5-6 drops of liquid soap & use a stirring rod to mix. 4. Pour soapy water from your cup into your beaker. 5. Continue to add soapy water by pipet until the top surface appears rounded. 6. Slowly add paper clips one at a time to the beaker keeping count of all paper clips that you add. 7. Stop adding paper clips to the beaker whenever water spills from the top. 8. Record your paper clip count. Compare the actual number of paper clips to the estimated number. Table 2 Cohesiveness of Soapy water Estimated Number of Paper Clips Actual Number of paper Clips Difference Questions: 1. How did your estimated number compare to your actual number? 2. What happened to the surface of the water as more clips were added? 3. What property of water was shown in Part A? 4. How is this property of water used in nature? 5. Explain why water shows surface tension. 6. Explain why water is a polar molecule and include a diagram of several water molecules in a drop of water. 7. In order to clean a surface, what must happen to surface tension? 8. What is the job of a surfactant? 9. Name a surfactant used in Part B? 10. Using your data from Part B, explain what proof you gathered in Part B to support your answer to question 9.