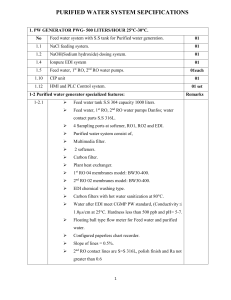
Foul Water Lab
advertisement
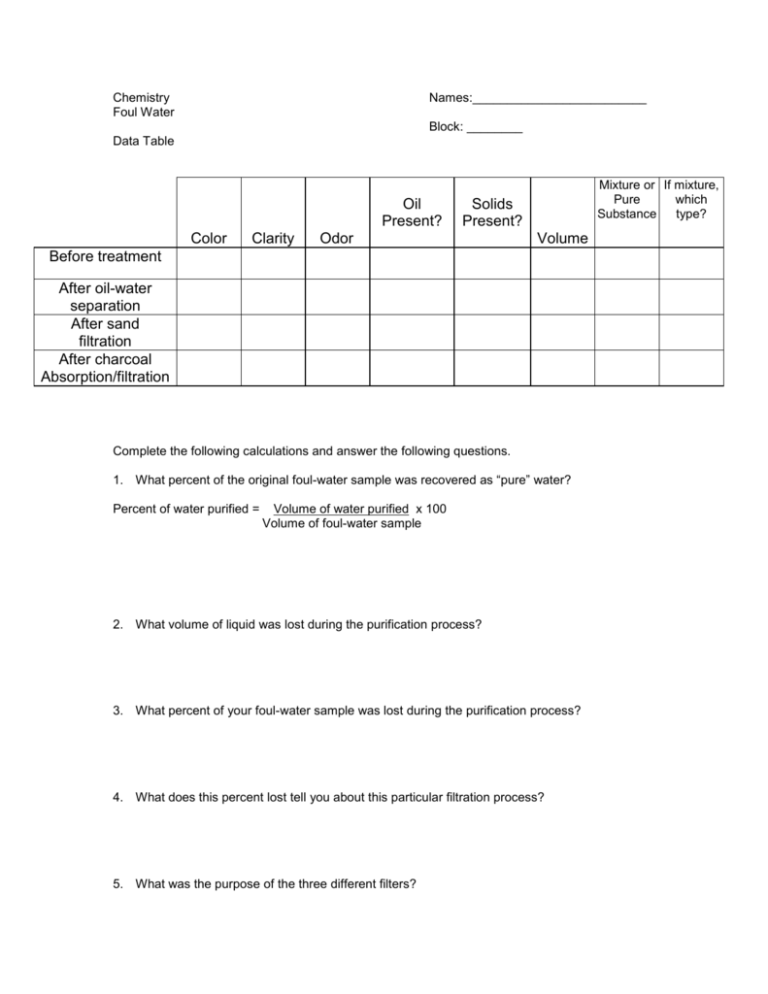
Chemistry Foul Water Names:_________________________ Block: ________ Data Table Oil Present? Color Clarity Mixture or If mixture, Pure which Substance type? Solids Present? Odor Volume Before treatment After oil-water separation After sand filtration After charcoal Absorption/filtration Complete the following calculations and answer the following questions. 1. What percent of the original foul-water sample was recovered as “pure” water? Percent of water purified = Volume of water purified x 100 Volume of foul-water sample 2. What volume of liquid was lost during the purification process? 3. What percent of your foul-water sample was lost during the purification process? 4. What does this percent lost tell you about this particular filtration process? 5. What was the purpose of the three different filters? A. pipet B. sand and gravel filter C. charcoal filter 6. Is your “purified water” sample “pure” water? How do you know? 7. Suggest how you might compare the quality of your water sample with that of other laboratory groups. That is, how can the relative success of each laboratory group be judged? Why? 8. How would you improve the water-purification procedures you followed so that a higher percent of purified water can be recovered? 9. What type of mixture is the final filtrate? How would you confirm this?