Richard F. Daley and Sally J. Daley
www.ochem4free.com
Organic
Chemistry
Chapter 17
Aromaticity
17.1 Benzene
868
Sidebar - Diamond, Graphite, and Buckyballs
17.2 The Stability of Benzene
874
17.3 Molecular Orbitals in Benzene
876
17.4 The Molecular Orbitals of Cyclobutadiene
17.5 Aromaticity
880
17.6 Hückel's Rule
882
17.7 Aromatic Ions
887
17.8 Naming Benzene Derivatives
891
17.9 Aromatic Heterocyclic Compounds
895
17.10 Polynuclear Aromatic Hydrocarbons
898
17.11 The Benzyl Group
900
Key Ideas from Chapter 17
901
872
879
Organic Chemistry - Ch 17
867
Daley & Daley
Copyright 1996-2005 by Richard F. Daley & Sally J. Daley
All Rights Reserved.
No part of this publication may be reproduced, stored in a retrieval system, or
transmitted in any form or by any means, electronic, mechanical, photocopying,
recording, or otherwise, without the prior written permission of the copyright
holder.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
868
Daley & Daley
Chapter 17
Aromaticity
Chapter Outline
17.1
Benzene
17.2
The Stability of Benzene
History of the origins of the structure of benzene
Evidence for the unusual stability of benzene
compared to other alkenes
17.3
Molecular Orbitals in Benzene
The molecular orbital structure of benzene
17.4
The Molecular Orbitals of
Cyclobutadiene
The molecular orbital structure of cyclobutadiene
17.5
Aromaticity
Definition of aromaticity and how the molecular
orbital structure of an aromatic molecule explains its
stability
17.6
Hückel's Rule
A statement of Hückel's Rule and a graphical method
for its application
17.7
Aromatic Ions
Some examples of aromatic anions and cations
17.8
Naming Benzene Derivatives
Nomenclature of derivatives of benzene
17.9
Aromatic Heterocyclic Compounds
The effect of placing a heteroatom in a conjugated ring
17.10
Polynuclear Aromatic Hydrocarbons
Aromatic hydrocarbons with multiple rings
17.11
The Benzyl Group
The special stability of the benzylic ion
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
869
Daley & Daley
Objectives
✔ Understand the basis for the structure and special stability of
benzene
✔ Recognize the differences between the molecular orbitals of
benzene and acyclic conjugated dienes
✔ Know the factors that make a compound aromatic, nonaromatic, or
antiaromatic
✔ Be able to apply Hückel’s rule and its graphical equivalent to
predict whether a particular compound is aromatic
✔ Know the IUPAC rules for naming benzene derivatives
✔ Recognize that the special stability of an aromatic compound
makes some molecules readily release a proton or a hydride ion in
order to become aromatic
✔ Distinguish whether or not a pair of nonbonding electrons on a
heteroatom is used to make a molecule aromatic
✔ Understand the basis for the special reactivity of a benzylic cation
I was sitting, writing at my text-book; but the work did
not progress; my thoughts were elsewhere. I turned my
chair to the fire and dozed. Again the atoms were
gamboling before my eyes. This time the smaller
groups kept modestly in the background. My mental
eye, rendered more acute by repeated visions of this
kind, could now distinguish larger structures of
manifold conformation: long rows, sometimes more
closely fitted together; all twining and twisting in
snake-like motion. But look! What was that? One of the
snakes had seized hold of its own tail, and the form
whirled mockingly before my eyes. As if by a flash of
lightning I awoke; and this time also I spent the rest of
the night working out of the consequences of the
hypothesis.
—Friedrich August Kekulé
(Describing how he arrived at his
proposed benzene structure)
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
870
Daley & Daley
I
An aromatic compound
has a conjugated ring
and a specific number
of π electrons. Benzene
is one example of an
aromatic compound.
n the second half of the 19th century, as chemists explored
the chemical components responsible for the odor of such
things as roses, vanilla, wintergreen, and almonds, they found that
each had a common molecular characteristic—a benzene ring.
Chemists then began calling all compounds that contain benzene ring
aromatic compounds. The name, aromatic, stuck for benzenecontaining compounds, not because of their odor, either pleasant or
unpleasant, but because of the presence of a benzene ring.
Benzene is a cyclic compound that consists of six carbon atoms
bonded together by σ bonds and a set of delocalized π bonds. All the
electrons associated with those π bonds delocalize over all six carbons.
Delocalized electrons in a cyclic conjugated system have a dramatic
effect on the stability of the molecule. In fact, this stability makes
aromatic molecules so unreactive that they do not follow the expected
reaction pathways that their structures suggest they should.
Aromaticity now refers to any compound that contains a ring of atoms
with a delocalized π molecular orbital involving every atom of the ring
and with 2, 6, 10, etc. electrons in the MO.
As chemists studied aromatic compounds, they found that they
possessed unexpectedly unique properties and reactivities. Benzene is
a very important example of the aromatic compounds; thus, most of
this chapter describes benzene and its derivatives. The rest of the
chapter looks at other aromatic compounds. Chapter 18 examines the
reactions of benzene.
17.1 Benzene
In 1825, Michael Faraday isolated a chemical compound that
condensed from illuminating gas, the fuel burned in gaslights. The
compound boiled at 80oC and had the unusually small hydrogen-tocarbon ratio of 1:1. This ratio corresponded to the empirical formula of
CH. In 1834, Eilhard Mitscherlich isolated the same compound by
heating benzoic acid, which is found in gum benzoin, in the presence of
lime. Mitscherlich also found that the compound had a 1:1 carbon-tohydrogen ratio and a molecular weight of 78. From this information he
derived the molecular formula of C6H6. Because of its source, he
named the compound benzin. It gradually evolved to the present name
of benzene.
For many years, chemists puzzled over the structure of
benzene. Although they proposed a number of structures, none fit the
experimental data. In the 1860s and 1870s, as chemists were able to
synthesize some of these proposed structures, they clearly proved that
these structures were not benzene. Two of the more interesting
structures are those proposed by Ladenburg and Dewar. Ladenburg
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
871
Daley & Daley
benzene, now called prismane because of its prism shape, isomerizes
to benzene when heated to 90o.
H
H
H
H
H
H
H
H
H
H
H
H
Ladenburg benzene
Dewar benzene
Another structure, proposed by Johann Josef Loschmidt in 1861,
describes benzene quite well. In his structure the large circle
represents the six carbon atoms, and the smaller circles represent the
hydrogen atoms.
Loschmidt's benzene
However, Loschmidt's proposal for the structure of benzene saw only a
very small circulation. He published a small book describing his work,
but he never traveled to meet with other chemists and never
published in the major scientific journals. Although his ideas were far
ahead of his contemporaries, he was so shy and quiet that his
contemporaries either didn't understand him or completely ignored
him.
In 1865, Friedrich August Kekulé proposed a structure for
benzene similar to those used so far in this book.
Kekulé benzene
Because Kekulé was more outgoing than Loschmidt, his proposal got
more attention. At first, the other chemists considered his structure
bizarre because double bonds had only been proposed in 1859 and had
not yet really been confirmed. However, in time, his structure became
widely accepted as a reasonable representation of benzene.
Ultimately, he received the credit for the proposal of a cyclic structure
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
872
Daley & Daley
for benzene with equal C—C bonds. Chemists call his representation
for benzene “Kekulé benzene.”
Kekulé's first proposed benzene structure indicated that
benzene was a cyclic conjugated triene. As a polyene with that much
unsaturation, chemists expected it to react in certain ways, but it
didn't. For example, according to Kekulé's structure, benzene should
undergo electrophilic addition, as do other compounds with double
bonds, but it doesn't. Instead it undergoes electrophilic substitution—
the topic of Chapter 18. In almost all reactions involving benzene, the
benzene ring stays intact. The aromatic carbon ring is so stable that
the substituents on the ring react, but not the ring itself. Any addition
reactions or any reactions that change the benzene ring require
extreme reaction conditions. Substitution reactions are the
characteristic reactions of benzene.
Br
Br2
FeBr3
Br
Br
Another problem with Kekulé's benzene structure is that two
different isomers of any 1,2-disubstituted benzene should exist, but
they don't. In one expected isomer, the two carbons with the
substituents would have a double bond between them. In the other
expected isomer, the two carbons with the substituents would have a
single bond between them. In real life, benzene reacts to form only one
disubstituted structure.
CH3
CH3
or
Br
Br
In response to this criticism, Kekulé proposed a second
structure that oscillated with the first structure. The second structure
was also a six-membered carbon ring with three single bonds and
three double bonds, but in this structure, the single bonds and the
double bonds had switched places. He suggested that this oscillation
was continual and took place so fast that the two isomers could not be
isolated.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
873
Daley & Daley
CH3
CH3
Br
Br
Today, chemists recognize that this sort of “oscillation” does not
take place—that there is no equilibrium between the two Kekulé
structures. Instead of “oscillating,” the compound is really a resonance
hybrid. In benzene, there are two resonance contributors with equal
energy.
To show that benzene is a resonance hybrid, chemists usually
represent benzene with a circle in the center instead of drawing
individual bonds. However, they do sometimes use Kekulé's structure
to show electron movement.
The hexagon with a circle inside indicates the delocalization of
the 6 π electrons in three occupied molecular orbitals. It also serves as
a reminder that all 6 C—C bonds have equal bond lengths. The
Kekulé structures, on the other hand, show alternating double and
single bonds. Typically double and single bonds have different lengths.
Measurements of the actual structure of benzene show that each of
the C—C bond lengths are the same (140 pm) and are midway
between the bond lengths of a single (147 pm) and a double bond (133
pm). As a result, both resonance contributors contribute equally to the
structure of benzene, which means that the two contributors have
equal energy.
Exercise 17.1
Benzene actually has three possible disubstituted isomers. Draw
them. How many different disubstituted isomers do the Dewar
benzene and Ladenburg benzene have? Draw them.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
874
Daley & Daley
SIDEBAR—Diamond, Graphite, and Buckyballs
About 200 years ago, chemists recognized that diamond and
graphite were pure allotropic forms of carbon. Allotropes are different
forms of the same element each having radically different properties.
But it wasn't until much later that they figured out the structures of
these two forms of carbon. Diamond consists entirely of sp3 hybridized
carbons. Each carbon atom bonds to four other carbon atoms. This
structure, shown below with one section highlighted, makes a very
rigid form of carbon and accounts for the fact that diamond is one of
the hardest of all materials.
The structure of diamond with a single unit highlighted.
Graphite, the most common form of pure carbon, has a
structure of sp2 hybridized carbon atoms arranged in a series of fused
benzene rings. The following illustration highlights one benzene ring
unit to help you see the arrangement. Graphite is really sheets of
carbon atoms that readily slide past one another on the electron
clouds of the π molecular orbitals. This arrangement makes graphite
soft and slippery—an excellent lubricant. Its black color and easy
spreadability also make it a perfect material for pencil “lead.”
The structure of graphite with a single unit highlighted.
In the early 1970s two research groups, one in Japan and the
other in Russia, simultaneously proposed a new form of carbon. They
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
875
Daley & Daley
suggested that it was a spherical molecule made up of 60 carbon
atoms arranged in rings: 12 five-membered rings and 20 sixmembered rings. To the Russian researchers, this new allotrope of
carbon looked much like a soccer ball, but it reminded their American
colleagues of the geodesic structures of Buckminster Fuller. Thus,
they named the new carbon allotrope buckminsterfullerene. The name
stuck, although usually they abbreviate the name to fullerene, or more
affectionately, to “bucky balls.”
Buckminsterfullerene, C60
Bucky balls were first isolated in the mid-1980s, but a practical
synthesis was not discovered until 1990. The Nobel Prize in chemistry
in 1996 was shared by Robert F. Curl, Jr. and Richard F. Smalley of
Rice University and Sir Harold W. Kroto of the University of Sussex,
Brighton, UK for their work in the synthesis and purification of bucky
balls. The synthesis is a simple process: heat a graphite rod to
temperatures above 1000oC in the absence of oxygen and collect the
soot that forms on nearby cool surfaces. Fullerenes are isolated from
this soot.
The discovery of bucky balls caught the interest of chemists
everywhere. Since 1990, chemists have made a variety of bucky ball
allotropes. The discovery and investigation of these molecules has
involved hundreds of researchers around the world working to
understand the chemistry of the fullerenes. At the present time, they
have made much progress.
A recently discovered allotrope of carbon consists of chains of
300-500 carbon atoms with alternating single and triple bonds. This
allotrope of carbon is synthesized by heating a thin graphite rod with
a laser under an inert atmosphere.
Structure of the chain of this allotrope of carbon
The carbon chains form a spiral, as the following illustration shows.
Little is known about this alkyne except that it is highly reactive and
conducts electricity.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
876
Daley & Daley
17.2 The Stability of Benzene
Aromaticity now refers to the unusual stability and low
chemical reactivity of compounds with the unique conjugation of cyclic
π bond delocalization. Benzene is the most common aromatic
compound. As discussed in Section 17.1, the alternating single and
double bonds of the Kekulé structure of benzene indicate that benzene
should be quite reactive, but it is not. Comparing benzene’s heat of
hydrogenation with the heats of hydrogenation of several similar
compounds that all hydrogenate to cyclohexane shows the effect of
aromaticity on benzene by allowing you to see their relative stabilities.
Cyclohexene readily hydrogenates to cyclohexane. The heat of
hydrogenation for this reaction is –28.6 kcal/mole. This amount is the
same heat of hydrogenation expected for any cis alkene.
H2
Pt
Ho = –28.6 kcal/mole
The heat of hydrogenation for 1,4-cyclohexadiene is –57.3 kcal/mole—
twice that of cyclohexene. 1,4-Cyclohexadiene has two isolated double
bonds, so this is the expected heat of hydrogenation.
H2
Pt
Ho = –57.3 kcal/mole
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
877
Daley & Daley
The two double bonds of 1,3-cyclohexadiene are conjugated, so
1,3-cyclohexadiene should have a lower heat of hydrogenation than
1,4-cyclohexadiene, and sure enough it does. The heat of
hydrogenation of 1,3-cyclohexadiene is –55.4 kcal/mole, which is 1.9
kcal/mole lower (more stable) than 1,4-cyclohexadiene.
H2
Ho = –55.4 kcal/mole
Pt
The Kekulé structure of benzene gives it the name of 1,3,5cyclohexatriene. From the Kekulé structure, the expected heat of
hydrogenation for benzene should be about three times that of
cyclohexene, or about –85.8 kcal/mole. However, when the reaction
was run, the actual heat of hydrogenation was –49.8 kcal/mole.
H2
Ho = –49.8 kcal/mole
Pt
Resonance energy is the
difference in the
calculated heat of
hydrogenation of a
conjugated compound,
assuming no resonance
or conjugation, and its
actual heat of
hydrogenation.
The heat of hydrogenation of benzene is 36 kcal/mole less
exothermic than expected based on three conjugated double bonds.
This difference, called the resonance energy, is due to the
delocalized π electrons. Figure 17.1 graphically shows the heats of
hydrogenation of these various compounds. Examination of this figure
clearly shows that the heat of hydrogenation of benzene is
considerably less than the hypothetical 1,3,5-cyclohexatriene and even
less than that of 1,3-cyclohexadiene. This difference gives you a good
picture of the relative stability of benzene.
1.9 kcal/mol
Conjugation
Energy
Energy
36 kcal/mol
Resonance
Energy
–28.6
kcal/mol
–57.3
kcal/mol
–85.8
kcal/mol
–55.4
kcal/mol
www.ochem4free.com
–49.8
kcal/mol
5 July 2005
Organic Chemistry - Ch 17
878
Daley & Daley
Figure 17.1. The heats of hydrogenation of cyclohexene, 1,3-cyclohexadiene, 1,4cyclohexadiene, the hypothetical 1,3,5-cyclohexatriene, and benzene.
According to resonance theory, whenever a molecule allows you
to draw significant resonance contributors, the hybrid molecule is
more stable than any of the individual resonance contributors would
be if they could exist. Although benzene has two equivalent resonance
structures, its stability cannot be explained by resonance alone. The
extra stability of benzene, when compared to the hypothetical 1,3,5cyclohexatriene, is due to its molecular orbitals.
Exercise 17.2
If the structure of benzene were actually 1,3,5-cyclohexatriene, the
carbon—carbon bonds would alternately measure 147 pm and 133 pm.
However, the structure could not exhibit resonance. The following
resonance structures violate a basic principle of resonance theory.
Explain.
17.3 Molecular Orbitals in Benzene
Benzene is a planar ring that consists of six sp2 hybrid carbon
atoms. Each of these sp2 hybrid carbon atoms possesses an
unhybridized p orbital. Because of the planar structure of the bonds
between the carbons, the p orbital of each carbon overlaps the
unhybridized p orbitals of its two adjacent carbon neighbors. This
overlapping of the p orbitals creates a set of six π molecular orbitals
that involves all of the carbon atoms in the ring and results in the
delocalization of the six available carbon electrons over the entire ring.
Although the MOs in benzene are cyclic instead of linear, like
those of the conjugated dienes and allylic systems covered in Chapter
16, they form according to the same principles. The six p atomic
orbitals of the six carbons form six MOs in the benzene π system. The
six MO energy levels are located equally above and below the energy
level of the starting atomic orbitals. The three lower energy level MOs
are the bonding MOs, so they fill with the electrons first. The three
higher energy MOs are antibonding and have no electrons in the
ground state.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
A nodal plane
symmetrically connects
individual nodes in a
molecular orbital.
879
Daley & Daley
An important consideration for drawing nodes in any π MO is
that each MO with nodes must have at least one plane of symmetry.
On both sides of that plane of symmetry the MO must have identical
features: the same number of nodes, or nodal planes, and the same
number of bonding, nonbonding, and antibonding orbital interactions.
To achieve symmetry in a cyclic MO, nodal planes must
symmetrically divide the orbital. Figure 17.2 shows the nodal planes
of the six π MOs of benzene.
Node
-
+
-
+
+
-
+
Node
+
6*
-
+
Node
-
+
Node
-
Node
+
+
4*
Node
+
-
+
+
-
-
+
+
5*
+
+
-
-
-
Node
+
+
-
+
+
-
2
Node
-
-
+
+
+
+
-
-
3
+
+
Node
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
+
880
+
+
+
+
-
Daley & Daley
+
1
-
-
-
Figure 17.2. The six π molecular orbitals of benzene showing the nodal planes of each
MO.
Multiple orbitals
having the same energy
are degenerate orbitals.
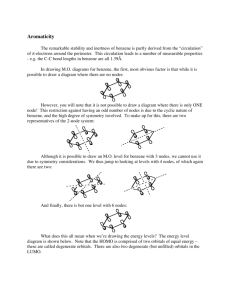
The number of perpendicular nodal planes in benzene ranges
from none in the lowest energy MO (π1), which has all bonding
interactions, to three in the highest energy MO (π6*), which has all
antibonding interactions. However, unlike the acyclic conjugated
polyenes, which increase in steps of one node per MO of increasing
energy, benzene increases the number of nodal planes. There are two
MOs of benzene (π2 and π3) each with one nodal plane and two (π4*
and π5*) with two nodal planes. When a molecule has two different
MOs with the same number of nodes, they usually have the same
energy level, too. When more than one orbital in one chemical species
have the same energy level they are called degenerate orbitals.
Figure 17.3 shows the various energy levels of benzene and
how they fill with electrons. Electrons fill the lowest energy level
orbital before filling higher energy level orbitals. The ground state of
benzene has six electrons in the π1, π2, and π3 MOs. These three MOs
have a lower energy level than the energy level of the isolated p
atomic orbitals from which they form. The six electrons in the three
delocalized π MOs have a much lower energy than they would if they
were in three localized π MOs. This accounts for the resonance energy
of benzene.
Energy
6
4
*
*
Antibonding
5
*
M Os
Isolated p
orbitals
2
3
Bonding
M Os
1
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
881
Daley & Daley
Figure 17.3. Energy diagram of the molecular orbitals of benzene showing the
distribution of electrons. The arrangement of the energy levels here corresponds with
the arrangement of the nodal planes in Figure 17.2.
17.4 The Molecular Orbitals of Cyclobutadiene
Although you can draw benzene-like resonance structures for
cyclobutadiene, experimental evidence shows that cyclobutadiene does
not have the stability of benzene. Instead, cyclobutadiene is very
unstable—much less stable than 1,3-butadiene. In fact, cyclobutadiene
is so unstable that it requires special conditions to isolate it. An
examination of the π MOs of cyclobutadiene helps explain its
instability.
The cyclobutadiene ring contains four sp2 hybrid carbons. Each
carbon has an unhybridized p orbital, and these p orbitals overlap to
form four molecular orbitals. The lowest energy MO (π1) contains all
bonding overlaps. The next two MOs (π2 and π3) are degenerate
orbitals with one symmetrically located nodal plane each. The final
MO (π4) has two nodal planes with all antibonding interactions.
Figure 17.4 shows these four MOs.
+
-
+
Node
+
-
4*
+
Node
+
-
+
-
+
+
+
2
Node
-
-
+
-
+
+
Node
www.ochem4free.com
5 July 2005
3
Organic Chemistry - Ch 17
882
+
+
+
-
Daley & Daley
+
-
1
-
Figure 17.4. The four π molecular orbitals of cyclobutadiene.
Cyclobutadiene has four π electrons to place in the above MOs.
Two electrons move into the π1 MO because the π1 MO is the lowest
energy molecular orbital. One electron then goes into each of the π2
and the π3 MOs. The π2 and π3 MOs are degenerate orbitals, and
according to Hund's rule, each degenerate orbital accepts one electron
before any can accept a second electron. These unpaired electrons are
very reactive; thus, making cyclobutadiene much more reactive than
benzene, which has all of its electrons paired. Figure 17.5 shows the
energy levels of cyclobutadiene.
Energy
*
4
Antibonding
MO
2
3
1
Isolated p
orbitals
Bonding
MO
Figure 17.5. Energy diagram of the molecular orbitals of cyclobutadiene showing the
distribution of electrons.
17.5 Aromaticity
Sections 17.2-17.4 discuss why benzene is aromatic and why
cyclobutadiene is not. This section further compares benzene with
cylobutadiene to summarize the aromatic characteristics of benzene
and to help you gain a better understanding of aromaticity.
Here is a list of characteristics that describe aromatic
compounds. To be aromatic, a compound must possess all of these
characteristics:
1. An aromatic compound is cyclic and contains delocalized MOs.
2. All the π electrons must be paired.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
883
Daley & Daley
3. The atoms in the ring are usually sp2 hybridized, although in
some cases they are sp hybridized. Each atom in the ring
contributes an unhybridized p orbital to form the delocalized π
MOs.
4. The structure of the compound is planar, or nearly planar.
Because the unhybridized p orbitals bond to form a continuous
ring of parallel π MOs, the ring must have a planar structure to
allow the unhybridized p orbitals to effectively overlap.
5. The π electrons are delocalized over the entire ring. This
delocalization lowers the energy of the molecule; therefore, the
compound is aromatic and has a resonance energy. As Section
17.6 points out, this is true only for systems with 2, 6, 10, and
so on in increments of four electrons in the π MOs.
Benzene meets all five criteria for a continuous ring of overlapping
orbitals. Thus, benzene is aromatic.
The comparison of benzene’s heat of hydrogenation and the
heat of hydrogenation of the hypothetical 1,3,5-cyclohexatriene shows
how the delocalization of the π electrons in benzene lowers its energy
level. A way to make similar comparisons for other aromatic
compounds is to look at the energy level of the cyclic compound with
an sp2 hybridized linear chain containing the same number of carbon
atoms and the same number of π electrons. Thus, when you compare
benzene with 1,3,5-hexatriene, benzene has much less energy.
Lower energy
More stable
An antiaromatic
compound is one that
meet criteria 1,3,4, and
5 for aromatic
compounds but has
more energy than its
acyclic counterpart
instead of less.
Higher energy
Less stable
Cyclobutadiene meets all of the criteria for a continuous ring of
overlapping orbitals. However, as stated in Section 17.4,
cyclobutadiene is very unstable. This lack of stability is due to the
unpaired electrons in π2 and π3. A comparison of cyclobutadiene with
its open chain counterpart, 1,3-butadiene, shows that cyclobutadiene
has more energy than 1,3-butadiene. Cyclobutadiene is
antiaromatic.
Higher energy
Less stable
Lower energy
More stable
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
A nonaromatic cyclic
compound usually has
a similar energy
content to its open
chain counterpart.
884
Daley & Daley
A cyclic molecule that does not have a continuous ring of
overlapping p orbitals is neither aromatic nor antiaromatic; it is
nonaromatic. For example, 1,3-cyclohexadiene is similar in energy
content to Z,Z-2,4-hexadiene. Thus, cyclohexadiene is nonaromatic.
Similar energy
Similar stability
17.6 Hückel's Rule
N is any integer value.
N=0, 1, 2, ….
In 1931 the German physicist Erich Hückel carried out a series
of mathematical calculations. With his calculations, he wanted to
figure out what made some compounds, such as benzene, aromatic and
other compounds that seemed so similar to benzene, like
cyclobutadiene, antiaromatic. His goal was to devise a way of
predicting whether or not a compound was aromatic.
Hückel limited his work to monocyclic molecules that seemed
to meet the criteria for an aromatic or an antiaromatic system. Within
that group of molecules, he postulated that the number of delocalized
π electrons contained in the molecule determined whether or not the
molecule was aromatic. He suggested that the number of π electrons
needed for aromaticity was 4N + 2 electrons. To get this number, he
looked at the molecule's molecular orbital arrangement and how these
MOs fill with electrons. Aromatic compounds with an even number of
p atomic orbitals forming delocalized π MOs arrange their π MOs with
one low energy level and one high energy level MO and pairs of
degenerate MOs in between. Aromatic compounds with an odd
number of p atomic orbitals forming delocalized π MOs arrange their π
MOs with one low energy level and pairs of degenerate MOs above
that. The MOs fill with electrons according to Hund's rule. The lower
levels fill first, and when there are degenerate MOs, both MOs fill
with one electron before either MO adds a second electron.
The molecular orbital levels in a π MO system can be identified
by quantum numbers much like the atomic orbitals in an element.
Each quantum number represents a shell of orbitals. The second level
shell has the 2s and three 2p atomic orbitals. These 2s and 2p atomic
orbitals are a shell of orbitals. Whenever an element has all of these
orbitals filled, the element is said to have a closed shell and is
especially stable. Neon has filled 2s and 2p orbitals and is an
especially stable and unreactive element.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
Hückel’s rule states
that an aromatic
compound must have
4N + 2 π electrons.
The polygon rule is a
graphical equivalent of
Hückel’s rule that
shows the relative
energy level of the π
electrons in a π MO.
This is also called a
Frost diagram.
885
Daley & Daley
A compound has a closed shell when it has the lowest energy π
molecular orbital filled plus 4N electrons where N is the number of
filled pairs of degenerate orbitals. The total number of π electrons in a
compound with a closed shell is 4N + 2 electrons. The closed shell
concept for π molecular orbitals is the basis for Hückel's rule, which
states that a compound must have (4N + 2) π electrons to be aromatic.
A molecule with 4N + 2 of π electrons is said to have a closed shell.
On the other hand, a compound with only 4N π electrons does
not have a closed shell. Such a molecule has an unpaired electron in
each of the highest energy occupied π MOs. Like the elements with
closed shells, molecules with closed shells are very stable and
unreactive. Elements with open shells are much more reactive than
are those with closed shells. Benzene has a closed shell of π MOs;
cyclobutadiene does not. The π MOs of benzene are very stable and
unreactive; those of cyclobutadiene are unstable and very reactive.
To find out how many delocalized π electrons a molecule must
have to be aromatic, solve the (4N + 2) formula with N equaling an
integer (0, 1, 2, 3, etc.). Hückel’s formula gives you 2, 6, 10, 14, etc.
delocalized electrons. When later researchers were able to make some
of these monocyclic molecules, they found a remarkable agreement
with Hückel's formula. Thus, (4N + 2) became known as Hückel's rule.
Hückel also found that systems with 4N π electrons are usually less
stable than their open chain counterparts. In other words, planar
monocyclic molecules with 4, 8, 12, etc., electrons are antiaromatic.
Another way to determine whether or not a compound is
aromatic is by using the polygon rule. The polygon rule quickly gives
you a picture of the relative energies, and thus the relative stability, of
the MOs of a conjugated monocyclic system. To use the polygon rule,
draw a regular polygon with the same shape as the molecule you wish
to examine. Orient your drawing with one vertex pointing toward the
bottom of your paper. Then draw a horizontal line through the middle
of the polygon. This line represents the energy level of the nonbonding
orbitals. Figure 17.6 shows how to apply the polygon rule to benzene
and cyclobutadiene. Next draw in the molecular orbitals by letting
each vertex represent one π molecular orbital.
Antibonding M Os
Nonbonding M Os
Bonding M Os
Figure 17.6. A polygon is used to derive the relative energies of the electrons in
benzene and cyclobutadiene. This method permits you to arrive at a similar result to
the orbital methods used earlier.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
886
Daley & Daley
Then, beginning with the bottom vertex as the fully bonding MO,
place the electrons at each vertex of the polygon. Remember to follow
Hund's rule. Figure 17.7 illustrates filling these figures with electrons.
By using this method, you arrive at the same conclusion that you
would by actually drawing the MOs. That is, benzene fills the lower
bonding MOs with pairs of electrons and cyclobutadiene does not.
These filled MOs give benzene a lower energy level than
cyclobutadiene. Thus, benzene is aromatic and cyclobutadiene is
antiaromatic.
Figure 17.7. Filling the benzene and cyclobutadiene polygons with electrons.
For a long time cyclooctatetraene was a puzzle to chemists.
With a total of eight π electrons, it does not meet Hückel's rule for
aromaticity, so it is not aromatic. It does meet the 4N number, and the
polygon rule (Figure 17.8) shows that it does not have a closed shell,
so it should be antiaromatic.
Figure 17.8. Applying the polygon rule to cyclooctatetraene. Like cyclobutadiene, this
molecule has two unpaired electrons. Thus, the polygon rule confirms that
cyclooctatetraene should be antiaromatic.
However, cyclooctatetraene does not act antiaromatic. In reactions, it
behaves like an ordinary polyene. Although not nearly as stable as
benzene, its stability is much greater than would be expected from an
antiaromatic compound.
It turns out that cyclooctatetraene is neither aromatic nor
antiaromatic because it is not planar. By using X-ray techniques,
chemists discovered that the bonds of cyclooctatetraene are
alternately long and short with lengths of 148 and 134 pm
respectively. These bond lengths give cyclooctatetraene a tub-shaped
conformation.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
887
Daley & Daley
The conformation of cyclooctatetraene
If cyclooctatetraene were to adopt a planar configuration, it would
actually lose stability rather than gain it. Thus, by taking up this tubshaped configuration, cyclooctatetraene is actually a nonaromatic
compound.
Exercise 17.3
Make a model of cyclooctatetraene. Estimate the angle between the
adjacent π molecular orbitals.
Exercise 17.4
There are three monocyclic structures with 10 π electrons. These three
structures have 0, 1, and 2 trans double bonds respectively. Each fits
the 4N + 2 rule. Using models, determine which are aromatic.
Annulene is a proposed
name for monocyclic
molecules with
alternating double and
single bonds.
Annulene is a proposed name for monocyclic molecules with
alternating single and double bonds. A number placed in brackets in
front of the word annulene indicates the number of atoms in the ring.
Thus, benzene is [6]annulene, and cyclooctatetraene is [8]annulene.
Chemists seldom use the annulene name for benzene and
cyclooctatetraene, but they use the annulene name almost exclusively
for the larger rings.
In the 1960s, Franz Sondheimer, as well as some other
chemists, produced a number of annulenes. Using these annulenes,
they tested and verified Hückel's rule. They found that most of the
annulenes with (4N + 2) electrons exhibited aromatic properties.
However, because annulenes are free to adopt nonplanar
conformations, none are antiaromatic. For example, the [14] and
[18]annulenes are examples of aromatic molecules. And the [12] and
[16]annulenes are examples of nonaromatic molecules.
[12]Annulene
(Nonaromatic)
[14]Annulene
(Aromatic)
[16]Annulene
(Nonaromatic)
www.ochem4free.com
[18]Annulene
(Aromatic)
5 July 2005
Organic Chemistry - Ch 17
888
Daley & Daley
Solved Exercise 17.1
Decide whether each of the following compounds is aromatic or nonaromatic.
a)
Solution
Each of the two rings is aromatic with 6 π electrons in each. Thus, the
compound is aromatic.
b)
CH2CH3
Solution
1,3,5-Cycloheptatriene has a total of six π electrons, but it is not aromatic.
Every atom in the ring does not contribute to the cyclic π molecular orbital.
The π molecular orbital is not continuous but is interrupted by the sp3
hybridized carbon atom bearing the ethyl group.
c)
Solution
This structure of [14]annulene has 14 π electrons, but it is not aromatic
because the ring is not planar. The hydrogens shown below would interfere
with each other if the molecule were planar.
HH
HH
Exercise 17.5
Classify each of the following compounds as aromatic, nonaromatic, or
antiaromatic.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
889
a)
b)
c)
d)
Daley & Daley
Sample solution
a) This molecule is a [14]annulene, but the molecule is not
planar because of interference between the hydrogens. As a result,
this [14]annulene is nonaromatic.
H H
H
H
If planar, these hydrogens would
be trying to occupy the same space.
17.7 Aromatic Ions
The resonance energy gained by an aromatic molecule is large
enough such that anytime a molecule can reasonably obtain (4N + 2)
electrons and become aromatic, it will. A number of cyclic species that
bear a positive or negative charge exhibit unusual stability that
suggests they are aromatic. These ions meet Hückel's rule, further
indicating that they are aromatic. This section describes the two most
common aromatic ions: the anion of cyclopentadiene and the cation of
cycloheptatriene.
Cyclopentadiene is unusually acidic for a hydrocarbon. Its pKa
is 16.6 (comparable to water and alcohols) in contrast to a pKa of 46
for cyclopentane. Because of its relatively high acidity,
cyclopentadiene readily converts to its anion when treated with a
moderately strong base. The NMR of the cyclopentadienyl anion shows
a singlet indicating that all five hydrogens are equivalent. From the
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
890
Daley & Daley
equivalency of the hydrogens, you can assume that all five carbons are
also equivalent.
OC(CH3)3
••
H
H
H
Cyclopentadiene
Cyclopentadienyl anion
Cyclopentadiene is not aromatic. It does not have the proper
number of π electrons. Plus, the π electrons that it does have are not
delocalized around the entire ring. The sp3 hybridized carbon of the
CH2 group has no available p orbital; thus, it blocks the cyclic electron
delocalization.
However, when cyclopentadiene reacts with a moderately
strong base, the CH2 carbon atom loses one proton and becomes sp2
hybridized. The loss of the proton produces a new p orbital occupied by
the two remaining electrons. The new p orbital then overlaps with the
p orbitals on either side of it. This overlap produces a ring with six
delocalized π electrons. The negative charge is distributed equally over
the five carbon atoms in the ring. Because the electrons are
delocalized, all the hydrogens, and thus all the carbons, are equivalent
as indicated by a singlet in the NMR spectrum.
-H
-
Because of the unusual stability of the cyclopentadienyl anion,
and because these six π electrons meet the criteria for Hückel's rule,
the cyclopentadienyl anion is considered to be an aromatic ion. The
polygon rule, illustrated for the cyclopentadieneyl anion in Figure
17.9, shows that the filled MOs are below the energy level of the
isolated p atomic orbitals.
Isolated p
orbitals.
Figure 17.9. The polygon rule applied to the cyclopentadienyl anion. The occupied π
MOs are below the energy level of the isolated p orbitals.
The unusually high acidity of cyclopentadiene occurs because
the anion is aromatic and has a resonance energy estimated to be
about 23 kcal/mole. This idea relates back to Chapter 5, where, as you
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
891
Daley & Daley
may recall, an acid's strength is proportional to the stability of its
conjugated base. Figure 17.10 shows the formation of the π orbitals of
the cyclopentadienyl anion.
H
–H
H
H
Figure 17.10. The π orbital picture for the conversion of cyclopentadiene to the
cyclopentadienyl anion.
Exercise 17.6
a) Using the polygon rule, explain the basis for the aromaticity of the
cyclopentadienyl anion.
b) Using the polygon rule, would you expect the cyclopentadienyl
cation to be aromatic? Would you expect it to be aromatic based on
Hückel's rule?
Cycloheptatriene, sometimes called tropylidene, is another
example compound that readily reacts to obtain (4N + 2) electrons to
become an aromatic cation. Cycloheptatriene has six π electrons, but
they are not fully delocalized over the entire ring because the CH2
group lacks a p orbital available to form a cyclic π MO. When treated
with a reagent that abstracts a hydride ion, cycloheptatriene converts
to the cycloheptatrienyl (or tropylium) cation. The cycloheptatrienyl
cation has an empty p orbital on the former CH2 group and that p
orbital then overlaps with the remaining π orbitals of the ring to give
rise to a ring with six delocalized π electrons. Because the electrons
are delocalized, a singlet in the NMR spectrum shows that all seven
hydrogen atoms are equivalent. If all seven hydrogen atoms are
equivalent, then all seven carbons are also equivalent with the
positive charge distributed equally over the seven carbon atoms.
–H
+
The six π electrons in the cycloheptatrienyl cation allow the
cation to meet the criteria for Hückel's rule. Thus, the
cycloheptatrienyl cation is an aromatic ion. Figure 17.11 shows the
polygon rule for the cation. Cycloheptatriene readily loses a hydride
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
892
Daley & Daley
because of the resulting cation's aromaticity and a resonance energy
estimated at 19 kcal/mol. Figure 17.12 shows the formation of the π
orbitals of the cycloheptatrienyl cation.
Isolated p
orbitals.
Figure 17.11. The polygon rule applied to the cycloheptatrienyl cation. The π MOs
are all lower in energy than the isolated p orbitals.
H
–H
H
H
Figure 17.12. The π orbital picture of the conversion of cycloheptatriene to the
cycloheptatrienyl cation.
Exercise 17.7
The following hydrocarbon has an unusually large dipole moment.
Explain how this dipole moment might arise.
17.8 Naming Benzene Derivatives
The various derivatives of benzene all have different names—
many of which do not readily indicate their functional groups. For
example, benzene with an amine group attached is called aniline, and
benzene with a methyl group attached is called toluene. Table 17.1
lists the names of some common aromatic compounds.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
Structure
893
Daley & Daley
Name
Structure
Name
COOH
NH2
Benzoic Acid
Aniline
COOCH3
CN
Methyl
benzoate
Benzonitrile
CH
CONH2
CH2
Benzamide
Styrene
CHO
CH3
Benzaldehyde
Toluene
CH3
O
CCH3
o-Xylene
Acetophenone
CH3
CH3
OH
CH3
Phenol
Mesitylene
CH3
OCH3
Anisole
Table 17.1. Names of some simple aromatic compounds.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
Note that for
disubstituted benzene
rings the terms, ortho-,
meta-, and para(abbreviated as o-, m-,
and p-) are often used
instead of 1,2-, 1,3-,
and 1,4- numbering
See Section 9.5, page
000.
894
Daley & Daley
The names in Table 17.1 are listed in priority sequence. For
example, an aromatic ring with an —OCH3 group is called an anisole.
However, when an aromatic ring contains both an —OCH3 group and
an —OH group, the —OCH3 group is called methoxy substituent
because —OH has a higher priority than the —OCH3 group. The
anisole name is used with lower priority substituents such as —CN or
–CH3 groups.
OCH3
HO
OCH3
NC
4-Methoxyphenol
(p-Methoxyphenol)
4-Cyanoanisole
(p-Cyanoanisole)
When two or more substituents are present on a benzene ring,
follow these steps to name the compound:
Step 1 Number the substituents to give them the lowest possible
numbers.
Step 2 If two or more of the substituents are the same, use the di-,
tri-, tetra-, etc., prefixes.
Step 3 List the different substituents in alphabetical order.
COOH
Cl
NH2
Br
Cl
3,4-Dichlorobenzoic acid
(not 4,5-dichlorobenzoic acid)
CH3
4-Bromo-2-methylaniline
(not 2-methyl-4-bromoaniline)
When a benzene ring is the substituent, use the phenyl group
name. When benzene is attached to a hydrocarbon chain, follow these
steps:
Step 1 Determine the parent name of the molecule based on the
larger structural unit. Name the molecule either as an alkyl
benzene or a phenylalkane.
Step 2 For unsaturated hydrocarbon groups, name the compound
as a phenylalkene.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
895
Daley & Daley
CH2CH2CH3
CH2CH2CH2CH3
Butylbenzene
CHCH2CH2CH3
4-Phenylheptane
CH3C
CHCH3
2-Phenyl-2-butene
Solved Exercise 17.2
Name the following using IUPAC nomenclature rules.
a)
O
OH
H2N
Solution
This molecule has both an carboxylic acid and an amine group. According to
Table 17.1, the carboxylic acid group has a higher priority than the amine.
Thus, the compound is a benzoic acid. The IUPAC name is 4-aminobenzoic
acid, but the compound is frequently called p-aminobenzoic acid. You might
recognize it as the compound called PABA on the labels of make-up and
sunscreen products.
b)
Br
Solution
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
896
Daley & Daley
This molecule has a bromine and a vinyl group. There is no special name for
a halobenzene, so the molecule is a styrene. The name is 3-bromostyrene or
m-bromostyrene.
c)
O
OH
OCH3
O
Solution
This molecule has an ester and a carboxylic acid. According to Table 17.1, the
carboxylic acid has a higher priority. The IUPAC name for this compound is
2-carbomethoxybenzoic acid. An ortho-dicarboxylic acid is called phthalic acid
so most chemists would call this compound methyl phthalate.
Exercise 17.8
Name the following compounds using IUPAC nomenclature rules.
a)
b)
COOH
Br
NH2
NO2
OH
c)
d)
CH2
CH
CH2CH3
CH2CH3
e)
f)
COOH
CH3O
OCH3
CCH3
NC
O
Sample Solution
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
897
Daley & Daley
b) 2-Amino-3-bromophenol
17.9 Heterocyclic Aromatic Compounds
A heterocyclic
compound contains a
ring with one or more
heteroatoms replacing
carbons in the ring.
A heterocyclic aromatic
compound is an
aromatic compound
with one or more
heteroatoms replacing
carbons in the ring.
So far, this book has covered only those aromatic compounds in
which the ring consists entirely of sp2 hybridized carbon atoms. In
many cyclic compounds, however, some other element replaces one or
more of the carbon atoms. These compounds are called heterocyclic
compounds. The elements that most frequently replace carbon in
heterocyclic compounds are nitrogen, sulfur, and oxygen.
Chemists
usually
illustrate
heterocyclic
aromatic
compounds with their Kekulé structures. Pyridine, pyrrole, furan,
and thiophene are examples of heterocyclic aromatic compounds:
N
••
Pyridine
••
N
H
••
••
••
••
O
Pyrrole
S
Furan
Thiophene
Pyridine has the same electronic structure as benzene. Pyrrole, furan,
and thiophene share the same electronic structure as the
cyclopentadienyl anion.
A strong indicator of pyridine’s aromaticity is its resonance
energy of 27 kcal/mole, an amount only slightly lower than benzene’s
resonance energy. The π molecular orbitals of pyridine also contain six
electrons, thus meeting Hückel's (4N + 2) rule for π electrons. Pyridine
gains its aromaticity without involving the nonbonding electrons
belonging to the nitrogen. These nonbonding electrons are in an sp2
orbital in the plane of the ring. Being sp2 hybridized and in the plane
of the ring means that this orbital does not overlap with the π
molecular orbitals of the ring. Figure 17.13 shows the orbital picture
of pyridine.
sp2 hybrid
orbital
N
•
•
Figure 17.13. The orbital structure of pyridine. The nonbonding electron pair in the
sp2 orbital of the nitrogen is not involved in the π MO of the ring.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
898
Daley & Daley
In pyrrole, the two nonbonding electrons on the nitrogen are in
a p atomic orbital. That p orbital overlaps with the π orbitals of the
two adjacent double bonds to complete the circle of π bonds and allows
these two electrons to delocalize over the entire circle of π bonds. With
the addition of these two electrons, the continuous ring of π molecular
orbitals contains six electrons and meets Hückel's rule. Pyrrole has a
resonance energy of 22 kcal/mole. Figure 17.14 shows the π orbital
structure of pyrrole.
Unhybridized p
orbital
••
N
H
Figure 17.14. The orbital structure of pyrrole. The nonbonding electrons in the
unhybridized p orbital of nitrogen are involved in the π MO of the ring.
By now you may wonder why some molecules use the
nonbonding electrons on the heteroatoms in the ring of π bonds and
others do not. They follow this simple rule: if a molecule can use a pair
of nonbonding electrons to become aromatic, it will. Compounds will
be aromatic if it is possible for them to be so because of the resonance
energy gained by being aromatic.
Exercise 17.9
Is pyridine a stronger or weaker base than pyrrole? Explain.
Furan and thiophene have identical structures, except that the
heteroatom in furan is oxygen and the heteroatom in thiophene is
sulfur. Their structures are similar to pyrrole in that both have a pair
of nonbonding electrons in a p orbital. Figure 17.15 shows the orbital
structure of furan. To be aromatic, the p orbital with the nonbonding
electrons overlaps with the adjacent double bonds to form the
aromatic π molecular orbitals. Furan's resonance energy is 16
kcal/mole, and thiophene's is 29 kcal/mole. The difference in their
resonance energies is because the sulfur atom in thiophene uses an
unhybridized 3p orbital to overlap with the π orbitals from the
adjacent carbon atoms. Because 3p orbitals are larger than 2p
orbitals, the electrons in the 3p orbital of sulfur are closer to the π
orbitals of the carbon than are the electrons in the 2p orbital of
oxygen. Being closer together results in a more effective orbital
overlap.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
899
Daley & Daley
Unhybridized p
orbital
••
O
•
•
Nonbonding
sp2 orbital
Figure 17.15. The orbital structure of furan. The structure of thiophene is very
similar to furan except that it contains a sulfur atom instead of the oxygen.
Exercise 17.10
Determine whether the following species are aromatic, nonaromatic,
or antiaromatic.
a)
c)
b)
••
••
N
S
••
••
S
••
O
••
••
d)
f)
e)
B
••
N
H
B
Sample solution
b) This compound is aromatic. Its structure is similar to the
structure of thiophene in that one pair of nonbonding electrons from
the sulfur is involved in the aromaticity. Sulfur's other pair of
electrons and the nonbonding electrons on nitrogen are not involved in
the aromaticity.
17.10 Polynuclear Aromatic Hydrocarbons
Polynuclear aromatic
hydrocarbons contain
two or more aromatic
rings each sharing a
pair of adjacent carbon
atoms between pairs of
rings.
Polynuclear
aromatic
hydrocarbons
(PAHs)
are
compounds that contain two or more fused aromatic rings. A
molecule with fused aromatic rings contains two or more benzene
rings that share two carbon atoms between them. Naphthalene is an
example of a fused aromatic compound.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
900
Fused aromatic rings
share two adjacent
carbon atoms between
two aromatic rings.
Daley & Daley
Naphthalene
Naphthalene consists of two aromatic rings with a total of 10 π
electrons. The p orbitals of the naphthalene carbons overlap around
the periphery of the two rings and, to a lesser extent, across the points
of the ring fusion. Naphthalene's resonance energy of 61 kcal/mole is a
strong indicator that the delocalization of the electrons around the two
rings produces an aromatic system. Figure 17.16 shows the orbital
structure of naphthalene.
Figure 17.16. The orbital structure of naphthalene.
See page 000 for a
structure of
[14]annulene.
A large number of fused benzene ring PAHs are aromatic.
Pyrene, for example, is tetracyclic. Although pyrene is aromatic, its
Kekulé structure, shown below, has a total of 16 π electrons. This
number does not meet Hückel's rule but, as you may recall, Hückel's
rule applies only to monocyclic compounds. However, if you ignore the
internal double bond and examine only the structure of the 14
electrons on the periphery, pyrene looks similar to [14]annulene. And
fourteen is a Hückel number. As expected, the internal double bond
reacts much like an ordinary double bond and undergoes addition
reactions. On the other hand, the π bonds on the periphery undergo
substitution reactions like any other aromatic ring.
This double bond
is not a part of the
delocalized resonance
Pyrene
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
A carcinogen is a
compound that causes
some form of cancer.
901
Daley & Daley
Some PAHs are among the most carcinogenic of compounds
known. Pyrene is a very potent carcinogen. Another carcinogen is
benz[a]pyrene. Over two hundred years ago, physicians made the
earliest association of a specific type of cancer with a specific
carcinogenic agent. Many chimney sweeps developed scrotal cancer,
and they recognized that something in the soot and tars from the
chimneys they cleaned caused it. Chemists now know that the
carcinogenic agent found in chimney soot and tar is benz[a]pyrene.
Benz[a]pyrene
The safety rules for researchers working with benz[a]pyrene
require that the researchers install special containment facilities in
their laboratory and that they follow specific types of procedures to
protect themselves from the carcinogenic dangers of benz[a]pyrene.
Yet, along with several other related carcinogenic compounds,
cigarette smoke contains benz[a]pyrene. Smokers routinely expose
their lung tissue to higher concentrations of benz[a]pyrene than the
law permits for laboratory workers.
17.11 The Benzyl Group
The benzyl group is a
benzene ring with a
CH2 group attached.
See Section 16.2, page
000 for more on the
allylic carbocation.
The benzyl group is a benzene ring with a —CH2 group
attached. A benzyl carbocation has a positive charge on the —CH2
carbon. The benzylic carbocation is quite similar to the allylic
carbocation in that both have an empty p orbital that can overlap with
the π MOs of an unsaturated system.
CH2
The benzyl carbocation
Both benzylic and allylic halides readily undergo nucleophilic
substitution. Primary and secondary benzylic and allylic halides
undergo substitution via an SN1 mechanism in a polar solvent. They
follow this mechanism because they are highly resonance-stabilized.
Following are resonance structures for the benzylic carbocation. Note
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
902
Daley & Daley
that the benzylic resonance stabilization is quite similar to the
resonance stabilization for the allylic cation shown in Chapter 16.
CH2
CH2
CH2
CH2
The resonance contributors for the benzylic carbocation.
Although there are four resonance contributors for the benzylic
carbocation, reaction only occurs at the CH2 group. Reaction at any
other positively charged carbon atom would lead to a nonaromatic
product and higher energy than reaction at the CH2 group. Thus, the
carbons in the ring do not react.
Figure 17.17 shows the MO picture of the benzylic carbocation.
As you may recall, the structure for a carbocation is planar with an
empty p orbital perpendicular to the plane of the sp2 carbon. This p
orbital overlaps with the π MO allowing electron density from the π
MO to be shared with the empty p orbital. This sharing of electron
density greatly increases the stability of the carbocation.
CH2
Figure 17.17. The molecular orbital picture of the benzylic carbocation.
Exercise 17.11
The following chlorides undergo solvolysis at the relative rates shown
below the formulas. Explain these results.
C6H5CH2Cl
1
C6H5CHClCH3
12.5
(C6H5)2CHCl
3750
(C6H5)3CCl
3.8 x 107
Key Ideas from Chapter 17
❏
Benzene is an unusual compound with a carbon to hydrogen
ratio of 1:1 and an extraordinary stability considering the large
number of units of unsaturation that it possesses.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
903
Daley & Daley
❏
The unusual stability of benzene results from the conjugation
of the double bonds around the six-membered ring. This type of
conjugation is called aromaticity.
❏
Each carbon in the benzene ring has an unhybridized p orbital,
and each of these p orbitals overlaps the p orbitals on the
carbon atoms adjacent to it. The continuous overlapping of p
orbitals forms a π molecular orbital system that encompasses
the entire ring.
❏
The π MO system for benzene actually contains six individual
molecular orbitals. Three of these MOs are filled, bonding MOs.
The remaining three are empty, antibonding MOs.
❏
Benzene has two pairs of degenerate orbitals. Degenerate
orbitals are a pair of orbitals that have exactly the same
energy. In benzene, the HOMOs are one pair of degenerate
orbitals, and the LUMOs are the other pair.
❏
Aromaticity requires a cyclic structure in which every atom is
either sp or sp2 hybridized and contributes a p orbital to the π
MO. The structure of the ring must be planar to maximize the
overlap of the p orbitals. It must also have (4N + 2) π electrons
included in its π MOs. Aromaticity increases the stability of the
molecule compared to a nonaromatic compound.
❏
A nonaromatic compound does not contain a continuous ring of
p orbitals and/or the ring does not adopt a planar conformation.
❏
An antiaromatic compound has 4N electrons in a cyclic
delocalized MO. Antiaromatic compounds are less stable than
aromatic and nonaromatic compounds.
❏
Hückel's rule is a statement of the number of electrons found in
the π molecular orbitals of a cyclic conjugated system. If they
contain (4N + 2) electrons and if the ring is planar, the
compound is aromatic. If they have 4N electrons and if the ring
is planar, the compound is antiaromatic. Nonplanar cyclic
compounds are usually nonaromatic regardless of the number
of electrons in the π molecular orbitals.
❏
The polygon rule is a graphical version of Hückel's rule. It can
be used to determine whether a compound might be aromatic
or antiaromatic.
www.ochem4free.com
5 July 2005
Organic Chemistry - Ch 17
904
Daley & Daley
❏
Use the name annulene to describe cyclic compounds with
alternating single and double bonds. In this system, another
name for benzene is [6]annulene.
❏
Aromaticity increases the stability of a molecule to such an
extent that when a compound can either add or subtract
electrons to become aromatic, it will do so. These ions are
called aromatic ions.
❏
A cyclic compound that contains one or more noncarbon atoms
in the ring is called a heterocyclic compound. Depending on the
structure of the orbitals, the nonbonding electrons may, or may
not, be available to complete Hückel's rule to form an aromatic
compound.
❏
Polynuclear aromatic hydrocarbons contain two or more fused
benzene rings. When benzene rings are fused, each pair of
benzene rings shares two carbons.
❏
A carbocation on a carbon attached to a benzene ring is called a
benzylic carbocation. Benzylic carbocations are resonancestabilized by the benzene ring similar to the way an allylic
carbocations are resonance-stabilized.
www.ochem4free.com
5 July 2005