Activity Series Lab: Metal Reactivity Experiment
advertisement

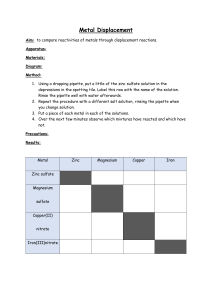
Lab 19: Activity Series Lab Materials: test tube rack and 6 test tubes small samples of 6 different metals copper (II) sulfate solution steel wool to remove oxidation from the samples Procedure: 1. Pour copper (II) sulfate solution into each test tube to a depth of approximately 1 cm. 2. Clean each of the metal samples with steel wool EXCEPT FOR LEAD to remove any oxidation. DON’T GET THEM MIXED UP! Lead dust in the room would be very bad since lead causes brain damage. 3. Put each metal sample in a different test tube, one at a time, so you can record your initial observations. 4. Record observations in your data table for approximately 5 to 10 minutes. Look for signs that a chemical reaction is occurring: gas bubbles being produced temperature change changes in color a precipitate forming 5. Rank the metals in order of reactivity: most reactive to least reactive. 6. Clean up – liquids in the waste beaker and solids in the trash. 7. Wash your hands and prepare for the class discussion. Questions 1. Explain why putting zinc into a solution of magnesium sulfate would NOT produce a reaction. 2. Use the activity series of metals and the activity series of nonmetals to predict whether the following reactions will occur. For the ones that do occur, tell which element would be oxidized and which element would be reduced. a) Al + AgNO3 b) NaF + Cl2 c) Zn + PbSO4 d) Cu + FeCl2 e) NiNO3 + Zn f) NaBr + Cl2 3. Explain how a more reactive metal like zinc can be used to “protect” a less reactive metal like iron. Give a practical example of this.