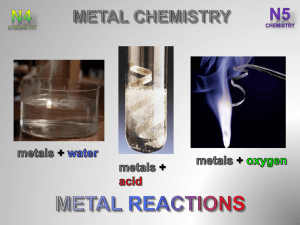
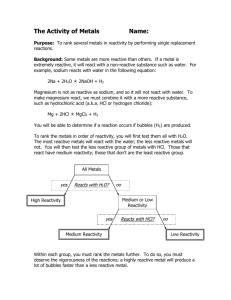
To investigate the relative reactivities of Ca, Mg, Zn and Cu based
advertisement

JSSS Student To investigate the relative reactivities of Ca, Mg, Zn and Cu based on their reactions with water and acid OC52 Remember what you learned in OC51 when you investigated the reaction between zinc and hydrochloric acid and tested for hydrogen. To jog your memory, answer the following in the spaces provided. 1. Write down the word equation and the chemical equation for that reaction. __________________________________________________________________________ __________________________________________________________________________ 2. Write down in order the names of these metallic elements: Ca, Mg, and Cu. __________________________________________________________________________ 3. Which two of these metals belong to the group known as the alkaline earth metals. __________________________________________________________________________ 4. What is the group number of the alkaline earth metals? __________________________________________________________________________ 5. Write down the test for hydrogen gas. __________________________________________________________________________ To help plan your investigation, answer the following 6. Would it be safer to react the metals first with water or to react them first with acid? __________________________________________________________________________ 7. How are you going to make the test fair? __________________________________________________________________________ 8. Write down the variables that should be kept the same (constant) for each metal. __________________________________________________________________________ 9. The change that we are going to make is to use a different each time we add water or acid. Now we will predict what we expect to happen 10. When we react the different metals separately and in turn with both water and acid we think that some will react than others. From what we will observe (see), we should be able to sort the metals into an order. We will place the most reactive metal first and the reactive metal with the other two in order in between. -1- JSSS Student The experiment Do not carry out the experiment until you have checked with the teacher that your method is safe. Safety glasses and gloves should be worn. Apparatus Graduated plastic droppers, distilled water, 3 M hydrochloric acid, Ca, Mg, Zn, Cu, well plate or spot plate. Possible setup Draw a labelled diagram of your setup in the space below. Procedure 1. From your group and class discussions, decide on the appropriate amount of each metal to be used 2. Because of the concentration of the acid (3 M), do not use more than 3 drops each time 3. Record your observations in a table Table recording data / observations Zn Mg Ca Cu Observations Observations Observations Observations No gas bubbles Water Hydrochloric acid -2- JSSS Student Looking for a pattern / trend in the results / observations 1. Which metal is the most reactive of the four? __________________________________________________________________________ 2. Explain why you think so __________________________________________________________________________ 3. Which metal is the least reactive of the four? __________________________________________________________________________ 4. Explain why you think so __________________________________________________________________________ 5. Which of the two metals left is the more reactive? __________________________________________________________________________ 6. Explain why you think so __________________________________________________________________________ Interpret and evaluate 1. Are you happy that your test was fair? __________________________________________________________________________ 2. How might it be improved? __________________________________________________________________________ __________________________________________________________________________ 3. Our conclusion is that the order of reactivity of these four metals, starting with the most reactive is __________________________________________________________________________ 4. Your group should now be ready to report back in a class discussion. -3-