Synthesis and Purification of Acetanilide from Aniline and Acetic
advertisement

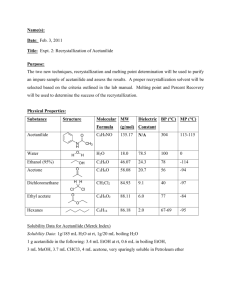
Synthesis and Purification of Acetanilide from Aniline and Acetic Anhydride Burns Introduction: Recrystallization is a method of purification used to isolate pure solids from a supersaturated solution, while leaving the impurities behind in the solvent. This technique involves a background with solubilities and is very useful to purify solids containing impurities. Recrystallization can be broken down into six steps11.) Choosing a suitable solvent 2.) Dissolving the compound in a minimum amount of solvent 3.) Removing insoluble impurities 4.) Crystallization 5.) Collecting and washing the collected solids 6.) Drying the crystals Polarity is the key to step number one. A solvent is chosen based on a polarity similar to the polarity of the compound you are attempting to purify1. To be successful in recrystallization the right solvent has to be used. An easy way to remember this is “like-dissolves-like.” A large factor that should be considered when deciding what solvent to proceed with, is making sure the solvent has a boiling point lower than the melting point of the solid, if not the solid will begin to melt and “oiling out” will occur, leaving poorly defined crystals1. Temperature plays a large role in recrystallization. For this experiment purification of acetanilide through recrystallization was performed. Acetanilide (C8H9NO) is an odorless white crystalline solid that is slightly soluble and nontoxic1. In the early 1900’s acetanilide was used in small doses to treat typhoid fever2, and has been proven to lower body temperature. It also used to be used in its treatment of obstinate vomiting, mostly due to nervousness, or gastric irritability3. Figure 1. Mechanism of Synthesis of Acetanilide 1 Synthesis and Purification of Acetanilide from Aniline and Acetic Anhydride Burns In this reaction, one C-O bond of acetic anhydride (C4H6O3) is broken, making the left carbon of the original structure a carbocation. A hydrogen proton from the amine group from the toxic aniline (C6H7N) attracts the now negatively charged oxygen molecule to form acetic acid (C2H4O2). The nitrogen from the original amine group then forms a bond with the carbocation to form acetanilide. The purpose of this experiment is to synthesize acetanilide, then follow this by an extraction workup to isolate as much product as possible. Once isolated the best solvent was determined to recrystallize acetanilide with. The success of this purification experiment is based on a melting point temperature, H NMR, and IR analysis. Experimental: Procedure one of this experiment was the synthesis of acetanilide. A sample containing 2mL of aniline, 15mL of deionized water, and 3mL of acetic anhydride was prepared. This reaction mixture was stirred with a stir plate for roughly thirty minutes. Around this time the product completely precipitated out of the solution. Crude acetanilide was then isolated through vacuum filtration using a porcelain Buchner funnel and a 125mL filter flask. The crude product was then allowed to dry on a filter paper, and a melting temperature of 112-1150C was observed. For procedure two a suitable recrystallization solvent was found. A heating mantle was used to determine what solvent would be best for recrystallization. Reaction tubes were prepared to test the solubility of acetanilide in four different labeled reaction solvents: water, ethanol, dichloromethane, hexanes. A 50mg portion of unknown was placed in each of the four test tubes along with .5mL of each solvent to their respective tubes. Gentle heating and careful observation was applied and recorded to determine the best solvent. One was looking for crystals that would dissolve in the heat, and reform in a beaker of ice water. Ethanol and dichloromethane both 2 Synthesis and Purification of Acetanilide from Aniline and Acetic Anhydride Burns dissolved the crystal instantly at room temperature. The hexane solvent didn’t dissolve the crystals at all, which left water, the solvent that didn’t dissolve the crystal in room temperature but did under heat, but also reformed the crystals under ice water. It was the best recrystallization solvent. The last step of the experiment was the purification of acetanilide. The remaining amount of crude product was recrystallized and isolated by vacuum filtration, and allowed to dry for seven days. The final dried product were dry, white, solid crystalline flakes, with a melting point of 112-1150C. Percent Yield: (grams of final recrystallized product/ grams of crude acetanilide) x100% (.742g/3.095) x100%= 23.97% NMR Data: 1 H NMR (60 MHz, C8H9NO) (ppm) 2.135 (s, 3H), 7.056 (m, 1H), 7.160 (m, 1H), 7.294 (m, 1H), 7.432 (m, 1H), 7.569 (m, 1H), 7.870 (s, 1H) IR Data: IR (cm-1) 3289.23, 3196.35, 3138.30, 3064.11, 1657.89 Crude Product: 3.095g Final Product: .742g Results and Discussion: A melting temperature point, NMR, and IR analyses were conducted to confirm the success of this experiment. The value recorded for the melting temperature of the final product for acetanilide of 1120 C was relatively close to the expected value of 1150C, but did match the mel-temp of the crude product. The difference could be due to impurities and possibly human error. The right solvent was chosen in the second procedure of the experiment because acetanilide, a polar molecule, dissolved in heated water, also a polar molecule, then recrystallized in cold water. A water solvent that was very close to the ideal recrystallization situation, where the compound should be completely soluble in the hot solvent, and quite 3 Synthesis and Purification of Acetanilide from Aniline and Acetic Anhydride Burns insoluble in the cold solvent. Hexanes a less polar molecule was not able to dissolve the compound, while DCM, and ethanol are both polar, they don’t have the magnitude of dipole moment that water has. Solubility depends on polarities and interactions of molecules between the solute and the solvent. An NMR analysis was also done to verify the results of the recrystallization process. The analysis matched the expected results, consisting of a multiplet in the 7-8ppm range that was the aromatic structure, and a single peak at 2.13 ppm that represented the methyl hydrogens. A hydrogen singlet that signified the presence of the N-H bond, was also represented by a small, flat, crest, just beyond the aromatic peaks having a value of 7.87 ppm. This NMR test was very comparable to the integral values observed shown in our lab manual. An IR was the last analysis performed for this experiment. An analysis check sheet for acetanilide predicted peaks in the 3300-3000 cm-1 absorption frequency range representing the aromatic C-H functional group. This was an accurate prediction as there were three peaks (3196.35cm-1, 3138.30cm-1, 3064.11cm-1) in that absorption frequency. An N-H functional group was predicted between 3500-3340cm-1 as well. An actual peak of 3289.23cm-1 was found in the analysis. Lastly a C=O functional group was predicted at 1690cm-1, this was not far off from the actual value of 1657.89cm-1. No big problems occurred during this experiment. Transferring some crude product, as well as the final crystals from watch glasses and wax paper and leaving some product were crucial to get the most final product. This explains why the recovery percentage of 24% was so low; some crystals either fell off, or didn’t transfer to the weighing paper. Even though the percent recovery was relatively low, this experiment still produced a .742g of product. 4 Synthesis and Purification of Acetanilide from Aniline and Acetic Anhydride Burns The results of the NMR, IR, and melting point were all pretty similar to the anticipated outcome. The only difference was that the mel-temp was 30 C under the recognized value. This difference can be attributed to impurities in the final product. NMR and IR analyses results were expected, and looked standard. Conclusions: This synthesis was successful and ended with excess product that, when tested show the results predicted in the pre-lab. Recrystallization was able to purify the product for the most part. There were probably still small traces of impurities in the final crystals, but after looking at the tests, they are considered to be typical. Choosing the correct solvent was critical to the lab, and water was definitely the right choice. Water dissolved the crystals in warm conditions and allowed them to recrystallize in cool conditions, exactly the circumstances needed for recrystallization. Overall this was a nice, fun, quick experiment. No recommendations are needed for repeating this process in the future. References: (1) Bortiatynski, Jackie. Lab Guide for Chemistry 203. Plymouth: Hayden- McNeil, 2013. N. pag. Print. (2) M'Mechan, J.C. "Acetanilide." The Cincinnati Lancet and Clinic 38.14 (1897): n. pag. American Periodicals Series Online. Web. <http://ezaccess.libraries.psu.edu/login?url=http://search.proquest.com.ezaccess.libraries.psu.edu /docview/88467135?accountid=13158>. (3)"Acetanilide for Vomiting." The Cincinnati Lancet and Clinic 33.24 (1894): n. pag. American Periodicals. Web. <http://ezaccess.libraries.psu.edu/login?url=http://search.proquest.com.ezaccess.libraries.psu.edu /docview/88460221?accountid=13158>. 5