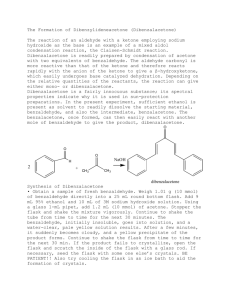
Chemistry 3719L – Week 10 Aldol condensation – Synthesis of
advertisement
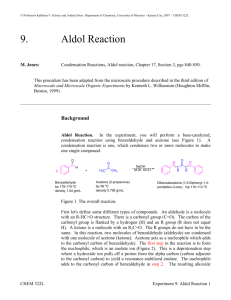
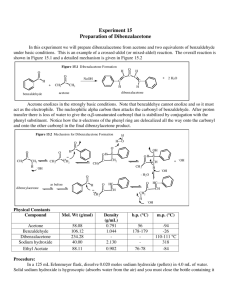
Spring 2004 Chemistry 3719L – Week 10 Aldol condensation – Synthesis of Dibenzalacetone Pre-lab reading from Zubrick: Chapter 13: Chapter 12: Whole Chapter – review recrystallization Pages 87-92 – review melting points Aims The aldol reaction is one of the most studied in all of Organic Chemistry and it also plays a major role in the formation of C-C bonds in Biochemistry (i.e. biosynthesis). This experiment highlights the formation of two new C-C bonds via deprotonation alpha to the carbonyl in acetone to generate the enolate, which then attacks the carbonyl of (nonenolizable) benzaldehyde. Overall the aldol reaction happens twice followed by loss of water to give the highly conjugated enone system of the product. Since two different carbonyl precursors are used this is known as a crossed aldol reaction. Reaction Acetone (1) has six alpha protons, which are all equivalent. Deprotonation with base generates the corresponding nucleophilic enolate, which then attacks the electrophilic benzaldehyde (2) to form a new carbon-carbon bond. This can happen again at the other alpha position such that one molecule of acetone has reacted with two molecules of benzaldehyde. Loss of two molecules of water is favored since the product is very highly conjugated dibenzalacetone (3). H + 1 H O O O H NaOH EtOH, H2O H H 3 2 Procedure • Dissolve sodium hydroxide (5g) in water (50 mL) and ethanol (40 mL) in a 250 mL Erlenmeyer flask. Make sure the mixture is at room temperature. • Mix benzaldehyde (5 mL) and acetone (1.9 mL) in a separate flask (small Erlenmeyer) and then add half of this mixture to the sodium hydroxide solution. Stir at room temperature for 15 minutes. • Add the rest of the acetone/benzaldehyde mixture and wash out the container with a couple of mL of ethanol, adding the washing to the reaction flask. • Stir the mixture at room temperature for a further 30 minutes then collect the solid product by vacuum filtration. Use the correct size of paper to avoid losing product. • Wash the product with distilled water (4 times, each with 50 mL of water) to remove any sodium hydroxide that remains in the product. • Air dry the solid for ~15 minutes then recrystallize the product using ~10 mL of ethanol for every 4g of product. • Record the weight of pure dibenzalacetone and obtain its melting point. • You should hand the product to your TA in a sealed, labeled test tube for grading. Synthesis Report = 10 pts Adapted from Williamson, Macroscale and Microscale Organic Experiments, Houghton Mifflin.