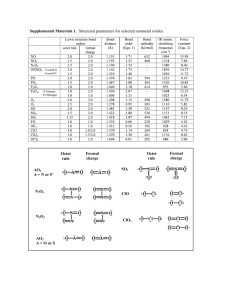
CS2 CCl4 Cl2O HF NCl3 CH2Cl2 HCN SF6 XeF4 SO3
advertisement

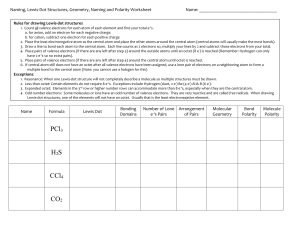
Complete the following table: Name Formula CS2 CCl4 Cl2O HF NCl3 CH2Cl2 HCN SF6 XeF4 SO3 Lewis Dot Number of Bonds Number of Lone e-‘s Pairs Geometry Shape Bond Polarity Molecule Polarity Naming, Lewis-Dot Structures, Geometry, Naming and Polarity Worksheet Name: __________________________________ Rules for drawing Lewis-dot structures 1. Count all valence electrons for each atom of each element and find your total e-‘s. a. for anion, add on electron for each negative charge. b. for cation, subtract one electron for each positive charge 2. Place the least electronegative atom as the central atom and place the other atoms around the central atom (central atoms will usually make the most bonds). 3. Draw a line to bond each atom to the central atom. Each line counts as 2 electrons so, multiply your lines by 2 and subtract those electrons from your total. 4. Place pairs of valence electrons (if there are any left after step 3) around the outside atoms until an octet (8 e-) is reached (Remember: Hydrogen can only have 2 e-‘s so no extra pairs). 5. Place pairs of valence electrons (if there are any left after step 4) around the central atom until octet is reached. 6. If central atom still does not have an octet after all valence electrons have been assigned, use a lone pair of electrons on a neighboring atom to form a multiple bond to the central atom (Note: you cannot use a halogen for this) Exceptions: 1. Resonance: When one Lewis-dot structure will not completely describe a molecule so multiple structures must be drawn. 2. Less than octet: Certain elements do not require 8 e-‘s. Exceptions include Hydrogen (duet, 2 e-) Be (4 e-) Al & B (6 e-). 3. Expanded octet: Elements in the 3rd row or higher number rows can accommodate more than 8 e-‘s, especially when they are the central atom. 4. Odd number electrons: Some molecules or ions have an odd number of valence electrons. They are very reactive and are called free radicals. When drawing Lewis-dot structures, one of the elements will not have an octet. Usually that is the least electronegative element. Name Formula PCl3 H 2S CF4 N2 CO2 BrF5 Lewis Dot Number of Bonds Number of Lone e-‘s Pairs Geometry Shape Bond Polarity Molecule Polarity