Lab: Electron Configuration Coloring Extra Practice
advertisement

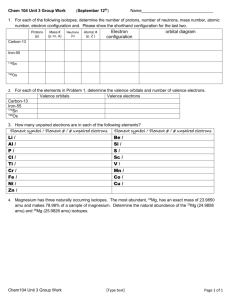
Name _______________________________ Electron Configuration Coloring Lab Extra Practice Answer the following questions using a periodic table. For each answer, provide a brief explanation of how you used the periodic table to determine the answer. 1. How many energy levels does an atom of copper have? 2. How many electrons can be found in an f-sublevel? How many orbitals can exist in an f-sublevel? 3. How many valence electrons does an atom of sulfur have? 4. How many electrons are in the 3d sublevel of nickel? 5. What is the maximum number of electrons that can be found in an s-sublevel? How many orbitals make up the s-sublevel? 6. How many energy levels does aluminum have? How many valence electrons? How many of the valence electrons are in a d-sublevel? In an s-sublevel? In a p-sublevel?