1 - Deans Community High School
advertisement

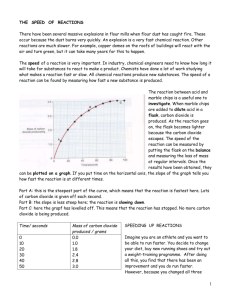
National 5 Chemistry Unit 1 Chemical Changes and Structure Sub Section 1 Rates of Reactions How to change the rate of a Chemical Reaction? Why can food be kept fresh for longer in a fridge? Why do chips cook faster than potatoes? As you found out in S2, chemical reactions occur at different rates (speeds). Activity 1.17 Rate of Chemical Reactions Copy and complete the following table. The reaction rate can be described as fast, medium or slow. Reactants SAFETY Reaction rate Take care with acids. A. Magnesium / water 1. Add a 1 cm piece of magnesium ribbon to a test-tube containing about 10 cm3 of water. Observe the rate of reaction. B. Barium chloride solution / sodium sulphate solution 2. Add a few drops of barium chloride solution to a test-tube containing about 10 cm3 of sodium sulphate solution. Observe the rate of reaction. C. Magnesium / dilute hydrochloric acid 3. Add a 1 cm piece of magnesium ribbon to a test-tube containing about 10 cm3 of dilute hydrochloric acid. Observe the rate of reaction. Copy and complete the following table. Use a √ to show the speed of the reaction. Chemical reaction food rotting limestone weathering burning of hydrogen firing caps in a toy gun bread toasting formation of oil iron rusting a cake baking a gas explosion Reaction rate Slow Mediu m Fast 2. How to measure the rate of a reaction? Discuss with your partner how you could measure exactly how fast a reaction is. Your teacher may show you a PowerPoint that shows some of the ways we can measure the speed of a reaction. Write your own notes about how the rate of a chemical reaction can be measured. You are going to use these methods in the next experiments. Factors Affecting the Rate of Reaction 3. Changing Particle Size Two experiments are to be carried out to see what affect particle size has on the speed of a reaction. The reaction to be investigated is that between calcium carbonate and hydrochloric acid. In this reaction carbon dioxide gas is produced and so we can get some idea of the reaction rate from the speed at which bubbles of gas are liberated. To make a fair comparison between the experiments only one condition must be changed. Samples of calcium carbonate chips and calcium carbonate powder are used. Other conditions like the concentration of acid and the temperature of the reactants must be kept the same in both experiments. Activity 1.18 Effect of particle Size SAFETY Take care with acids. 1. Measure 25 cm3 of hydrochloric acid into each of two small beakers. 2. Weigh out 2 g of powdered calcium carbonate into one test-tube and 2 g of calcium carbonate chips into the other. 3. Add the powdered calcium carbonate to the acid in one beaker and the chips to the acid in the other beaker. Write a short note on what you did and observed. You can draw diagrams to help you. Answer the following questions 1. In a chemical reaction, what happens to the reaction rate as the particle size of a reactant decreases? 2. How does a decrease in the particle size of a reactant affect its surface area? 3. Hence, as the surface area of a reactant increases what happens to the reaction rate? Now take Notes 1.3, complete it and paste it into your notes. Activity 2 Set up the Rates of Reaction Experiment – Effect of particle Size (experiment sheet available) 4. Changing Concentration Collect an Effect of Concentration Experiment sheet and follow the instructions on the sheet. Use the sheet to help you write up the experiment in your notes- make sure you include Introduction/ Aim Method Diagram Results Conclusion 4. Change in Temperature Collect an Effect of Temperature Experiment sheet and follow the instructions on the sheet. Use the sheet to help you write up the experiment in your notes- make sure you include Introduction/ Aim Method Diagram Results Conclusion Now take Notes 1.3, complete it and paste it into your notes. Summary Reaction rates - an explanation Answer the following questions 1. Does sawdust burn faster or slower than wooden logs? Explain. 2. Why is an oxygen / acetylene mixture used in a burner to cut metal rather than an air / acetylene mixture? 3. Which take longer to cook, large or small potatoes? Explain. 4. Why can food be kept fresh for longer periods when stored in a fridge? 5. Why is pure oxygen used in oxygen tents to speed up the recovery of patients in hospital? 6. Does the exhaust of a car rust faster or slower than the bodywork? Explain. 7. Why do plants grow faster in warm weather compared to cold weather? Extension Can you explain how reaction rate increases when particle size, concentration and temperature are changed in terms of particles? Discuss your ideas with the rest of the class and write a note about this. 5. Following the Course of a Chemical Reaction Often it is useful to follow the course of a chemical reaction rather than just looking at the starting point and finishing point. If we can follow how quickly a reactant is being used up or how quickly a product is being formed, we can compare different reactions more successfully. Collect NOTES 1.4 and stick this into your notes. Complete the sheet. Answer the following questions 1. Using the data in Appendix 1.4, for R, draw a graph of 'concentration / mol l-1 ' against 'time / s ‘. 2. Now, for P, draw a curve of 'concentration / mol 1 ' against ' time / s ' on the same set of axes as your first curve. Label each curve clearly. Stick the graph into your notes. 3. What happens to the concentration of R as the reaction proceeds? 4. What happens to the concentration of P as the reaction proceeds? 6. Average rate of a reaction Collect NOTES 1.5 and stick this into your notes. 1. Use the graph to calculate the average rate of reaction, in terms of the concentration of R, over the periods: (a) 10 - 20 s, (b) 20 - 40 s. (Remember the units.) 2. In terms of the concentration of P, now calculate the average rate of reaction over the periods (a) 0 - 10 s, (b) 10 - 20 s, (c) 20 - 40 s. (Remember the units.) 3. What happens to the reaction rate as the reaction proceeds? 4. Sketch a graph of 'rate of reaction / mol 1-1 s-1 'against 'time / s ' assuming the reaction goes to completion. (It is not necessary to use graph paper here.) 5. During the course of a reaction, at what point is the rate most rapid? 7. Investigating the reaction between calcium carbonate and hydrochloric acid. 8.1 Recording the Rate of a Reaction (i) Read the following information and carry out the experiment making sure you follow the instructions carefully. Consider the reaction between marble chips and dilute hydrochloric acid. Carbon dioxide gas is produced in this reaction. The course of the reaction can be followed by monitoring the rate at which this gas is liberated. The reaction can be carried out in an open-necked flask and the mass of the reaction mixture will decrease as the reaction proceeds. The loss in mass will be identical to the mass of gas liberated. In this case the mass of the reaction mixture can be measured at intervals during the reaction. Present your results in a table with the headings: Time / min Mass of flask + contents / g Mass of gas released / g 1. Using a measuring cylinder, measure out 50 cm3 of 2 mol-1 of hydrochloric acid into a conical flask. Place a loose plug of cotton wool in the neck of the flask. 2. Weigh out 10 g of marble chips into a small beaker and leave the beaker and its contents on the balance pan. 3. Place the conical flask on the balance pan and record the total mass of the apparatus. 4. Remove the cotton wool plug from the flask. Add the marble chips to the acid and at the same time start a stop clock. Return the cotton wool plug to the neck of the flask and keep the empty beaker on the balance pan. 5. Record the mass of the apparatus every 30 s for about 15 minutes. Collect NOTES 1.7 and stick this into your notes. Answer the following questions 1. Suggest other methods of monitoring a chemical reaction and thus determining its rate. 2. Write a balanced equation for the reaction between marble chips (calcium carbonate) and dilute hydrochloric acid. 3. Describe the way the course of this reaction was followed. 4. Draw a labelled diagram of the apparatus which was used. 5. Draw a graph of 'mass of carbon dioxide / g ' against ' time / min’ and stick it into your notes. 6. Why does the curve level off after some time? 7. Calculate the average rate of reaction over the periods (a) 0 - 3 mins, (b) 3 - 6 mins, (c) 6 - 9 mins. (Remember the units.) 8. From your graph, estimate the time it takes for (a) all the acid to react, (b) half the acid to react. 9. Explain why the time taken for all the acid to react is not exactly twice the time taken for half the acid to react. 8.2 Recording the Rate of a Reaction (ii) For the same reaction, the gas could be collected in a graduated gas syringe or in a graduated tube over water. The rate at which the gas is liberated could then be monitored by measuring its volume at various times during the reaction. Activity 1.22 Carry out the experiment as in Activity 1.21 but using changes in volume to measure the rate of reaction. 1. Suggest another way to follow the course of the reaction between marble chips and dilute hydrochloric acid. 2. For this method, draw a labelled diagram of the apparatus you used. 3. Draw a graph of 'volume of carbon dioxide / cm3 ' against 'time / min' and stick it into your notes. 4. Calculate the average rate of reaction over the periods (a) 0 - 3 min, (b) 3 - 6 min, (c) 6 - 9 min. (Remember the units.) 8. Catalysts Catalysts are substances which speed up chemical reactions without being 'consumed' by the reaction. Oxygen and water are the products of hydrogen peroxide, H202(1), decomposition. Activity 1.23 Investigating the Effect of a Catalyst 1. Add hydrogen peroxide to a test-tube to a depth of 2 cm and test for the production of oxygen. 2. Add a pinch of manganese(IV) oxide to the hydrogen peroxide. Note the difference in the reaction rate and test for oxygen production. Answer the following questions 1. What is meant by a catalyst? 2. Write an equation for the decomposition of hydrogen peroxide. 3. How can the presence of oxygen be shown? 4. At room temperature, is sufficient oxygen liberated to give a positive test? 5. What can be concluded about the rate of decomposition of hydrogen peroxide at room temperature? 6. What was added to the hydrogen peroxide to act as a catalyst? 7. What then happened to the rate of hydrogen peroxide decomposition and was there sufficient oxygen liberated to give a positive test? 10. Types of Catalyst Catalysts can be divided into two main types, namely, heterogeneous and homogeneous. A heterogeneous catalyst is one in which the reactants are in a different state to the catalyst. If the catalyst and reactants are in the same state, then the catalyst is described as homogeneous. Activity 1.24 Types of Catalyst In the following experiment, cobalt ions are used to catalyse the reaction between potassium sodium tartrate and hydrogen peroxide. 1. Add water to a test-tube to a depth of 2 cm. To this add a spatulaful of potassium sodium tartrate and shake the test-tube until the solid dissolves. 2. Add a few drops of cobalt(II) chloride solution until the mixture has a distinct pink colour. 3. Gently warm the mixture in a bunsen flame (do not let it boil or get too hot). 4. To the heated mixture, add a few drops of hydrogen peroxide solution and note any colour change. Does the original pink colour return? 5. Add a few more drops of hydrogen peroxide. Do the same colour changes take place? Read the following information about types of catalysts. Catalysts are widely used in industry. Nickel, for example, is used to hydrogenate vegetable oils in the manufacture of margarine. Iron is used in the manufacture of ammonia by the Haber process while platinum catalyses the oxidation of ammonia to nitrogen dioxide in the Ostwald process. In the manufacture of poly(ethene) and polyesters, the catalysts are titanium(IV) oxide and nickel oxide respectively. The alcohol, methanol, can be produced industrially by the direct combination of carbon monoxide and hydrogen in the presence of chromium(VI) oxide as catalyst. Cracking of long chain hydrocarbons is achieved using aluminium silicate which is present in clays. Answer the following questions; 1. What is meant by a heterogeneous catalyst? 2. What is meant by a homogeneous catalyst? 3. Which substance can catalyse the decomposition of hydrogen peroxide? (Look back to Activity 1.23 if necessary.) 4. Is this catalyst a homogeneous or a heterogeneous catalyst? Explain. 5. Which ions can be used to catalyse the reaction between potassium sodium tartrate and hydrogen peroxide? 6. Is this catalyst homogeneous or heterogeneous? Explain. 7. What colour are the ions which catalyse the reaction? 8. Does the catalyst change chemically during the reaction? How can you tell? 9. Is the catalyst regenerated at the end of the reaction? How can you tell? 10. From the information on the previous page and from any text book you have access to, give some examples of industrial catalysts, the reactions which they catalyse and classify them as heterogeneous or homogeneous. Present the information in a table 11. Catalyst poisoning Collect NOTES 1.6 and complete it. 12. Enzymes The catalysts for the reactions which take place in the living cells of plants and animals are called enzymes. The enzyme amylase, which is found in both saliva and the small intestine, helps convert starch into maltose. The maltose is converted to glucose in the small intestine with the aid of another enzyme called maltase. Gastric enzymes such as pepsin and trypsin convert proteins to peptides in the stomach and small intestine. The peptides are then converted to amino acids in the small intestine by the action of enzymes called peptidases. Lipase is another enzyme found in the small intestine. It hydrolyses fats forming fatty acids. As well as catalysing the reactions in biological systems, enzymes are also important in a number of industrial processes. For example, zymase, in yeast, catalyses the fermentation of sugars to alcohol, protease enzymes are added to detergent powders to assist stain removal and can be added to meat to make it more tender, papain can remove the 'haze' in brewing and cellulase is used to make jeans look more faded and worn. Catalase is an enzyme found in many different fruits and vegetables. Like manganese(IV) oxide it catalyses the decomposition of hydrogen peroxide to form water and oxygen. Activity 1.25 Enzymes 1. Set up a rack of five test-tubes and add hydrogen peroxide to a depth of 2 cm to each. 2. Add small pieces of different fruits and vegetables to four of the test-tubes and keep one as a control. Note the difference in reaction rate in each test-tube. Answer the following questions 1. What is meant by an enzyme? 2. Give examples of enzymes which are involved in digestion and the reactions which they catalyse. Present the information in a table. 3. Give examples of enzymes used in industry and the reactions which they catalyse. Present the information in a table. 4. Which enzyme catalyses the decomposition of hydrogen peroxide? 5. Describe how to investigate the concentration of this enzyme in different fruits and vegetables. 6. Suggest why this experiment only gives a rough comparison of the concentration of catalase. Summary Tasks 1. Draw a spider diagram showing what you have learned about how to change the rate of a chemical reaction. There are 4 factors to consider. 2. Choose one of the factors that have been investigated and draw a - cartoon strip OR – leaflet that explains how Reaction Rate is affected by your chosen factor.