Practical 7 - A-level chemistry
advertisement

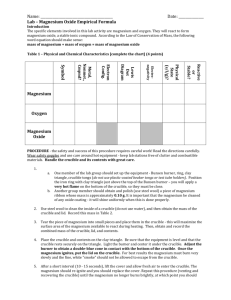
YEAR 12 PRACTICAL 7 – GRAVIMETRIC ANALYSIS Determining the empirical formula of magnesium oxide Magnesium reacts with oxygen to produce magnesium oxide. By reacting a known mass of magnesium with oxygen and weighing the mass of magnesium oxide produced, the empirical formula of the oxide can be deduced. Method 1. Weigh a crucible with its lid on a 2dp balance. 2. Add a small piece of magnesium ribbon to the crucible and weigh it again. 2. Place the crucible into a clay pipe triangle and heat with a roaring flame with the lid off. As soon as the magnesium starts to react, place the lid on the crucible to prevent smoke escaping. After the reaction stops, heat for one more minute to ensure the reaction is complete. 3. Allow the crucible to cool, and then weigh it again. Analysis 1. Deduce the mass of magnesium used in the experiment. 2. Deduce the mass of magnesium oxide produced, and hence the mass of oxygen added. 3. Deduce the moles of magnesium and oxygen present in the oxide. 4. Hence deduce the empirical formula of the oxide. Evaluation 1. Deduce the overall percentage apparatus error, given that the percentage error in the mass balance is ±0.01 g and two critical measurements were made. 2. Using the first analytical method, work out the difference between the moles of magnesium and the moles of oxygen, and express as a percentage of the moles of magnesium. 3. Compare this to you the apparatus error.