CHEN_354_Syllabus-Fa..

CHEN 354 Fall 09 Perla B. Balbuena
Catalog Data: CHEN 354. Chemical Engineering Thermodynamics II . 3 Credits.
Instructor: Prof. Perla B. Balbuena, JEB 240, balbuena@tamu.edu
, 845-3375
Course Times:
Office hours:
MWF 1:50pm-2:40pm, BICH 107
3:00-4:00 pm Thursdays, please call or e-mail ahead to schedule a visit outside these times.
Course Website: http://che.tamu.edu/orgs/groups/Balbuena/index.htm
Instructor Website: http://che.tamu.edu/balbuena
Textbook: J. M. Smith, H. C. Van Ness and M. M. Abbott, Introduction to
Chemical Engineering Thermodynamics (7 th
ed. McGraw-Hill,
NewYork, 2005).
Reference Books: R. C. Reid, J. M. Prausnitz & B. E. Poling, The Properties of Gases and Liquids, 4 th
ed., McGraw-Hill, 1987
S. M. Walas, Phase Equilibria in Chemical Engineering,
Butterworth, 1985
Prerequisites: CHEN 205, MATH 308, and CHEN 320 (or registration therein)
Skill Prerequisites : You are expected to have the ability to:
Know the first and second law of thermodynamics and the concepts of internal energy, work, entropy, enthalpy, and free energy
Write mass and energy balances of non-reactive systems
Solve quadratic and cubic equations
Know how to differentiate and integrate functions
Use computer software for linear and nonlinear algebraic equations, and linear and nonlinear regression.
Course Outcomes: By the end of the course, you should be able to apply phase and reaction equilibrium concepts to chemical engineering problems, specifically
:
Solve phase equilibrium problems. Understand the concepts of chemical potential and fugacity. Select appropriate models for calculating phase equilibria of specific gas, liquid and solid mixtures. Calculate bubble point pressure; bubble point temperature; dew point pressure; dew point temperature; flash calculations.
Interpret experimental data and select appropriate models to describe thermodynamics of mixtures with multiple phases. Apply phase equilibrium concepts to engineering problems.
Work effectively in teams. Work effectively in homework assignment teams and develop problem solving skills.
Exams: There will be three non-cumulative exams during the term and a cumulative final exam. Exam I is scheduled on September 28 th
, Monday; Exam II on October 26 th
,
1
CHEN 354 Fall 09 Perla B. Balbuena
Monday, and Exam III on November 16 th
, Monday. The final exam is scheduled on
December 15 th
, Tuesday, from 3:30pm to 5:30pm.
Homework: Homework sets extracted from the textbook will be assigned once a week, but they will not be graded. However, 1-2 problems from a different textbook will be assigned per week and they will be graded.
Grading Policy: Exam I
Exam II
Exam III
Final Exam
Homework
Tentative Grading Scale*:
100 – 86 A
85 – 78 B
20%
20%
20%
25%
15%
77 – 68 C
67 – 55 D
Below 55 F
*NOTE : This grading scale is tentative and may change. The minimum score needed for a certain grade may decrease, but will not increase.
MAKE-UP POLICY: Make-up exams will be given only for a university approved excuse in writing. Consistent with University Student Rules, students are required to notify the instructor by the end of the next working day after missing an exam.
Otherwise you will lose your right.
2
CHEN 354 Fall 09 Perla B. Balbuena
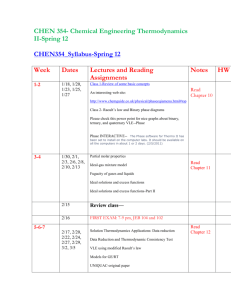
Tentative Course Schedule
Date
9/18, 9/21, 9/23,
9/25
Topic
8/31, 9/2, 9/4, 9/7, Introduction to vapor-liquid equilibrium; the Phase Rule;
9/9 Duhem's theorem; simple VLE models
9/11, 9/14, 9/16 Chemical potential, phase equilibrium conditions, partial properties
Ideal-gas mixtures, fugacity, ideal solutions, excess properties
9/28 First Exam
Liquid-phase properties from Vapor Liquid Equilibria 9/30, 10/2, 10/5,
10/7, 10/9, 10/12,
10/14, 10/16
(VLE) data, models for Excess Gibbs energy, property changes of mixing
10/19, 10/21,
10/23
10/26
10/28, 10/30,
11/2, 11/4, 11/6,
11/9, 11/11, 11/13
11/16
Thermodynamic properties and VLE from equations of state
Second Exam
Stability; liquid-liquid equilibria, solid liquid equilibria, vapor-liquid-liquid equilibria.
11/18, 11/20,
11/23, 11/25,
11/30
12/4, 12/7
Third Exam
Reaction coordinate, equilibrium criteria, equilibrium constants , temperature and pressure effects on the equilibrium constant, multireaction equilibria, fuel cell
Molecular theory of fluids, microscopic view on thermodynamic functions, statistical mechanics, hydrogen bonding and charge-transfer complexing
12/15 Final Exam
Reading
Ch.10
Ch.11
Ch 11
Ch.12
Ch.14
Ch 14
Ch 13
3
CHEN 354 Fall 09 Perla B. Balbuena
Policies and Procedures:
Americans with Disabilities Act (ADA) Statement
The Americans with Disabilities Act (ADA) is a federal antidiscrimination statute that provides comprehensive civil rights protection for persons with disabilities. Among other things, this legislation requires that all students with disabilities be guaranteed a learning environment that provides for reasonable accommodation of their disabilities. If you believe you have a disability requiring an accommodation, please contact the Department of Student Life, Services for Students with Disabilities in Cain Hall, Rm. B118, or call
845-1637.
Academic Integrity Statement
“An Aggie does not lie, cheat, or steal or tolerate those who do.”
As commonly defined, plagiarism consists of passing off one’s own the ideas, work, writings, etc., which belong to another. In accordance with this definition, you are committing plagiarism if you copy the work of another person and turn it in as your own, even if you should have the permission of that person. Plagiarism is one of the worst academic sins, for the plagiarist destroys the trust among colleagues without which research cannot be safely communicated. If you have questions regarding plagiarism, please consult the latest issue of the Texas A&M University Student Rules, under the section “Scholastic Dishonesty”. Please see the Honor Council Rules and Procedures on the web at http://www.tamu.edu/aggiehonor.
Course Outcomes and ChE program outcomes
Course Outcomes ChE Program
Outcomes
1, 2, 3, 5, 11 1. Solve phase equilibrium problems.
Understand the concepts of chemical potential and fugacity.
Select appropriate models for calculating phase equilibria of specific gas, liquid and solid mixtures.
Calculate bubble point pressure; bubble point temperature; dew point pressure; dew point temperature; flash calculations.
Interpret experimental data and select appropriate models to describe thermodynamics of mixtures with multiple phases.
Apply phase equilibrium concepts to engineering problems.
2. Work effectively in teams.
Work effectively in homework assignment teams and develop problem solving skills.
6, 7
4