Quantitative electrolysis
advertisement

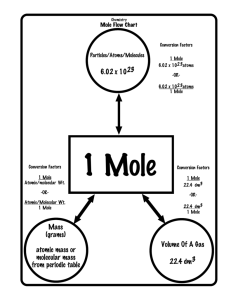
11.7 Quantitative Electrolysis The aim of the investigation is to use the electrolysis of dilute sulphuric acid to confirm the theoretical quantity of electricity required to produce one mole of hydrogen. 1. Theoretical Quantity The ion-electron equation for the formation of hydrogen gas is: 2H+(aq) + 2e- H2(g) The equation shows that two moles of electrons are needed to produce one mole of hydrogen gas. The quantity of charge needed = = 2 x 96,500 193,000 C 2. Electrolysis Experiment The purpose of this activity is to record the time taken to produce a measured volume of hydrogen during the electrolysis of dilute sulphuric acid using a constant current. The apparatus was set up as shown above. The current was passed through the solution for about 1 minute to allow the electrodes to become saturated with gas. The current and timer were switched on at the same time and left until 20cm3 of hydrogen had been collected. A current of 0.5 A was maintained by adjusting the variable resistor throughout the experiment. Results Current 0.5 A Time 318 s Volume Produced 21.5 cm3 3. Calculation and Conclusion Given that the molar volume of hydrogen is 24.08 l mol-1 the quantity of charge required to produce 1 mole can be calculated using the following steps: Step 1: Calculate the charge passed in the experiment. Q = It = 0.5 x 318 = 159 C Step 2: Work out the charge needed to make 24.08 litres (24,080 cm3) of hydrogen. Volume Charge 21.5 cm3 159 C 1 24,080 159 21.5 159 x 24,080 21.5 Charge needed for 1 mole of H2 = 178,080 C This agrees with the theoretical value (within experimental error).