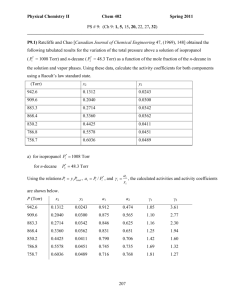
Solution Set #9
advertisement
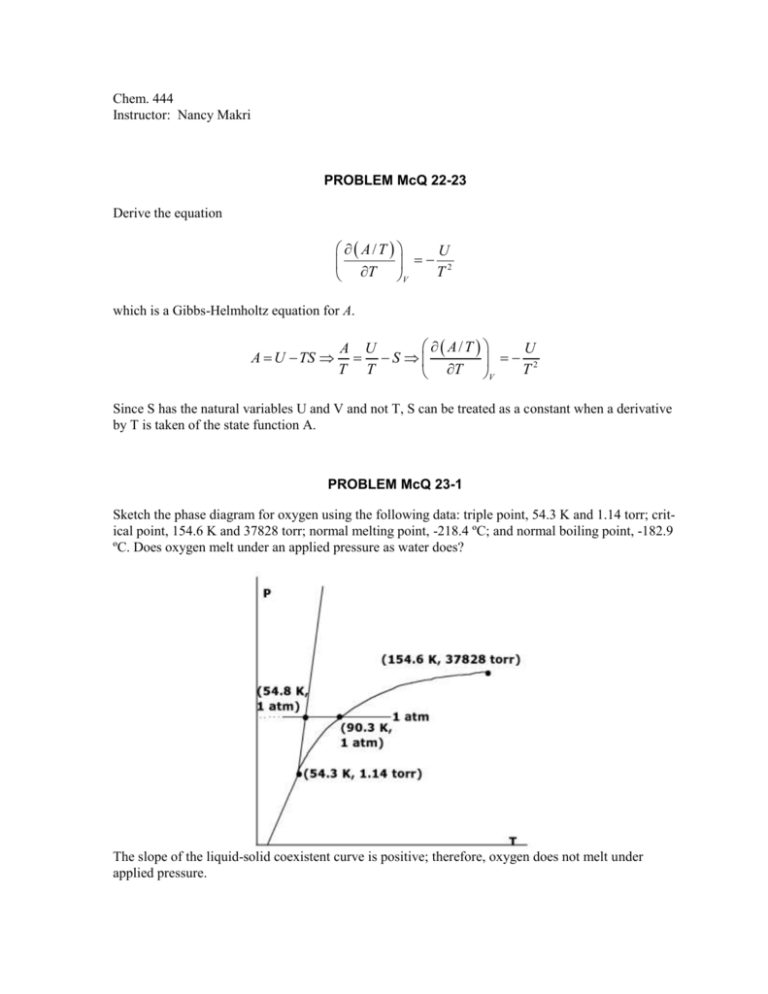
Chem. 444 Instructor: Nancy Makri PROBLEM McQ 22-23 Derive the equation A/T U 2 T T V which is a Gibbs-Helmholtz equation for A. A U TS A/T A U U S 2 T T T T V Since S has the natural variables U and V and not T, S can be treated as a constant when a derivative by T is taken of the state function A. PROBLEM McQ 23-1 Sketch the phase diagram for oxygen using the following data: triple point, 54.3 K and 1.14 torr; critical point, 154.6 K and 37828 torr; normal melting point, -218.4 ºC; and normal boiling point, -182.9 ºC. Does oxygen melt under an applied pressure as water does? The slope of the liquid-solid coexistent curve is positive; therefore, oxygen does not melt under applied pressure. PROBLEM McQ 23-2 Sketch the phase diagram for I2 given the following data: triple point 113ºC and 0.12 atm; critical point 512 ºC and 116 atm; normal melting point 114 ºC; normal boiling point 184 ºC; and density of liquid > density of solid. PROBLEM McQ 23-20 Determine the value of dT/dP for water at its normal boiling point of 373.15 K given that the molar enthalpy of vaporization is 40.65 kJ·mol-1, and the densities of the liquid and vapor are 0.9584 g·mol-1 and 0.6010 g·L-1, respectively. Estimate the boiling point of water at 2 atm. dP v H dT T vV T V f Vi Tm 1 1 dT T vV dP v H v H v H f i Which after plugging in the appropriate values yields, dT 0.2750K·L·kJ -1 = 27.50K·bar -1 = 27.12K·atm -1 dP Making the following crude approximation, dT T T dT dP T + T 373.15K + (27.12K·atm -1 )(1 atm) = 400.27K = 127.12 C dP P P PROBLEM McQ 23-27 The molar enthalpy of vaporization of water is 40.65 kJ·mol-1 at its normal boing point. Use the Clausius-Clapeyron equation to calculate the vapor pressure of the water at 110 ºC. The experimental value is 1075 torr. Assuming that the enthalpy change of vaporization is constant over the temperature range considered, the Clausius-Clapeyron equation can be integrated. P2 d ln P P1 v H RT 2 T2 dT T 2 T1 to get the integrated form, which with some algebra yields the following answer. ln P2 v H 1 1 P2 1070 torr . P1 R T1 T2 Note: P1 760 torr at 100 C as this is the boiling temperature at STP and 760 torr is ambient pressure.