Gases Review Activity
advertisement

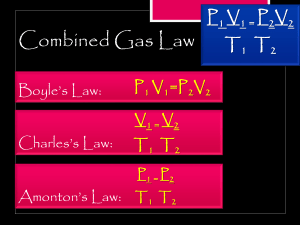
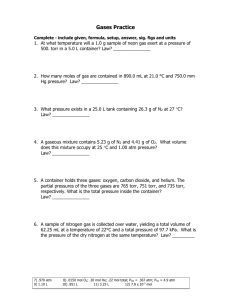
Boyles Law 1. Write the equation for Boyle’s Law. 2. What is the value of V2 if the initial pressure is doubled? 3. A container expands to 14.0 L as the pressure changes from 5.0 mmHg to 1.75 mmHg. What was the original volume? (4.9 L) Kinetic Molecular Theory (KMT) 4. According to the KMT, all particles move in this manner. 5. What kind attraction exists between gas particles, according to the KMT? 6. According to the KMT, all collisions are of this kind. Charles’ Law 7. What is the formula for Charles’ Law? 8. What is the value of T2 if the initial volume is doubled? 9. The temperature on a 1250. mL sample of nitrogen gas changes from 185°C to 45.0°C. What is the new volume of the gas? (868 mL) Guy-Lussac’s Law 10. Write the equation for Guy-Lussac’s Law. 11. What is the value of P2 if the initial temperature is halved? 12. The temperature in a rigid container changes to 207°C while the pressure changes from 1520 torr to 2125 torr. Calculate the original temperature. (343 K) Combined Gas Law 13. Write the equation for the Combined Law. 14. If pressure is held constant, the combined gas law becomes what law? 15. A gas takes up a volume of 17.0 L, has a pressure of 2.3 atm and a temperature of 299 K. If I raise the temperature to 350 K and lower the pressure to 1.5 atm, what is the new volume of the gas? (31 L) Ideal Gas Law 16. Write the formula for the Ideal Gas Law. 17. What units must you use for pressure, volume, temperature, and amount of substance? 18. What quantity is represented by n, in the Ideal Gas Law? 19. If I have 720 milliliters of Krypton gas held at a pressure of 2584 torr and a temperature of 225 K, how many moles of gas do I have? (0.133 mol) Avogadro’s Principle 20. How many liters of a gas are in 1 mol of a gas at STP? 21. How many moles of Cl2 are in 35.0 L of Cl2 at STP? (1.56 moles) 22. How many grams of CO2 are in 7.75 L of CO2 at STP? (15.2 g) Dalton’s Law 23. The total pressure in a container of gases is equal to what? 24. A mixture of helium, argon and neon has a total pressure of 4.30 atm. What is the partial pressure of neon if the partial pressure of helium is 1.25 atm, and the partial pressure of argon is 1.05 atm? (2.00 atm) Graham’s Law 25. Arrange the following in order of decreasing rate of effusion: C3H8 , O2, Xe, H2 26. Which gas effuses faster, C2H6 or O2? Why? **Gas Stoichiometry** (HP Only) 27. How many liters of oxygen gas can be produced at 30.0°C and 813.2 torr from the decomposition of 100.0 g potassium chlorate? 2 KClO3 ∆ 2 KCl + 3 O2 (28.3L)