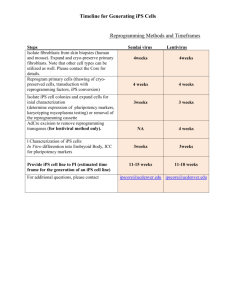
Studying Hematopoietic Contribution to Non
advertisement

Studying Hematopoietic Contribution to Non-Hematopoietic Tissues during Normal Development Tsai-Ching (Jack) Hsi Mentor: Maksim V. Plikus Cellular reprogramming can be defined as a conversion from one specific cell type into another. Recently, several advances have placed in vitro cellular reprogramming, such as induced pluripotent stem cell generation, at the forefront of regenerative medicine. Conversely, lack of insight into underlying molecular mechanisms, shortcomings of existing experimental models, and complex tissue morphology have placed in vivo reprogramming under intense scrutiny. With this in mind, we propose a novel model to study in vivo reprogramming in a clear, quantifiable fashion. The elastic cartilage of the mouse pinna is composed mainly of large chondrocytes with little extracellular matrix. Cells, arranged in a nearly two-dimensional array, appear as “pebbles in a mosaic”, allowing for quantification down to a single-cell resolution. Using cell lineage tracing based on genetic Cre-lox technology, we can trace a fraction of ear chondrocytes back to a hematopoietic (aka blood) lineage. These potentially hematopoietic-derived chondrocytes form discrete clusters and show a distinct distribution along the medial-to-lateral axis, with significantly more clusters along the lateral side, indicating spatially-dependent cell reprogramming efficiency. Quantification analysis of these lineage traced chondrocytes has revealed that approximately 17.5% of mouse adult ear cartilage is hematopoietic-derived. To our knowledge, this is the first time that hematopoietic reprogramming has been shown to contribute to an unrelated tissue under normal physiological conditions and in a developmentally significant manner. Taken together, the external ear cartilage allows for clear quantification of the cell lineage reprograming events, making it an ideal model to study this fundamental biological process in vivo.