Lab - Boiling Point & Density
advertisement

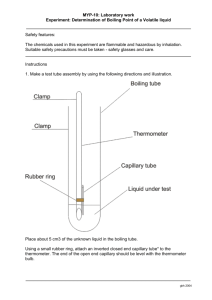
CHEMISTRY - Laboratory Logbook Entry LABORATORY EXERCISE: Density and Boiling Point Determination of an Unknown liquid Lab Instructions: PAGE 1 Objectives: 1. To identify an unknown liquid using two physical properties: the density and boiling point. Discussion and Review: In this experiment we will examine additional physical properties of a liquid. Two of the more important physical properties of pure substances are their density and boiling point. The boiling point of a liquid is the temperature at which that liquid is converted to a gaseous state. Boiling point is formally defined as the temperature at which the vapor pressure of the liquid becomes equal to the pressure at the surface of the liquid. The boiling point of a liquid can change if the pressure at the liquid's surface changes. Since pure substances have a distinct boiling point, boiling points are sometimes used to determine the purity of substances. Density is an intensive property that does not depend on the amount of the substance that is used. Boiling points are recorded in the Handbook of Chemistry and Physics, and can be found in the sections titled "Physical Constants of Organic Compounds" and "Physical Constants of Inorganic Compounds". PAGE 2 PART 1 Purpose: Determining the Densities of an unknown liquid DISCUSSION Density is an important property of matter. By itself, or in conjunction with other properties, density can be used to identify substances. Density is defined as the quantity of matter in a given unit of volume. This relationship, expressed mathematically, is: Density = mass (g) or D = m Volume (mL) V You will be expected to use the measuring skills and techniques developed in earlier lab sessions and in the first part of this experiment to find the mass and volume of different substances. You will use these data to calculate the density of these substances. Materials Used: Beaker 1-Disposable pipette Electronic balance 10-mL graduated cylinder Unknown liquid PROCEDURES: Part I. Densities of Liquids at Room Temperature. 1. Clean and dry your 10 mL graduated cylinder. 2. Weigh the cylinder carefully and record its mass to the nearest hundredth. 3. Go to the hood area, and pour out approximately 10 mL into a dry-cleaned beaker. 4. Go back to your lab station and pour some of the unknown in your graduated cylinder. Record the volume on your data table. (Be sure to take your measurement from the bottom of the meniscus.) 5. Weigh the cylinder and liquid on the balance. Record the mass. 6. Pour some more of the unknown liquid from your beaker to the volume that was previously recorded in step 4. Record the new volume. 7. Weigh the cylinder and the new volume on the balance. Record the mass. 8. Using the disposable pipette, add some more of the unknown liquid to the previous volume. Record the new volume. 9. Weigh the cylinder and the new volume. Record the mass. 10. Clean out the graduated cylinder and beaker with tap water and dry the beaker. PAGE 3 PART II Purpose: To determine the boiling point of one unknown liquid. Materials Used: Striker 1 Unknown liquid 1-2 small rubber bands Thermometer beaker closed-end capillary tubes (melting point tubes) 10-12 mm diameter test tube Laboratory Burner Ring stand Iron ring Wire gauze PROCEDURES: Part II. Boiling Point 1. Make a test tube assembly by using the following directions and illustration. a. Place about 1 mL of liquid 1 in a 10-12 mm diameter test tube. b. Using a small rubber band, attach a thermometer to the outside of the test tube. The thermometer bulb should be even with the test tube's bottom. c. Insert an inverted closed end capillary tube into the test tube. 2. Make a water bath assembly by using the following directions and illustration. a. Half fill a 100 mL or larger beaker with warm tap water. [Note: a water bath is used if the boiling point of the material is expected to be less than the boiling point of water; otherwise, an oil bath is needed.] b. Place the above test tube assembly in the water bath so that the surface level of the alcohol in the test tube is beneath the surface level of the water bath. c. Place the beaker on the wire stand and, stirring frequently to insure even heating, carefully heat the water bath with your heat source until the water bath boils and a rapid stream of bubbles continuously emerges from the capillary tube. d. Remove the heat source and begin observing the stream of bubbles. e. When the last bubble emerges from the capillary tube, record the temperature. 3. Reheat the water bath and repeat the cooling process two more times. Record the temperature reading after each trial, and average all three trials. DATA ANALYSIS Data Table #1: Unknown #1 Mass(g), volume (mL), and density (g/mL) measurements Mass of Empty Cylinder (g) Mass of Liquid & Cylinder in Grams (to nearest 0.01g) Mass of Liquid in Grams (to nearest 0.01g) Volume of Liquid in mL (to nearest 0.1mL) Density of Liquid in g/mL (to nearest 0.1g/mL) CALCULATIONS for Unknown #1 A) Mass of the Liquid (g) = [Mass (g) Liq + cylinder] - [Mass (g) empty cylinder] B) Density (g/mL) = mass (g)/volume(mL) C) Averaged Density (formula needed) = __∑x_ = sum of density (g/mL) n number of trials TABLE # 1: Mass (g) and Volume (ml) for UNKNOWN #1 Trials 1 2 3 Mass (g) Volume (mL) GRAPH #1 1. Construct a Mass (y axis) vs Volume (x axis) graph for unknown #1 using the measurements from the data table. 2. Calculate the slope (page R 76 in text for chemistry) of the line graphs. (Show the formula used, and calculations on the graph for each line) Be sure to clearly label the graphs for the unknown. PART II DATA TABLE #2: Boiling point of Unknown 1 (ºC) Trials BP (ºC) - Unknown 1 1 2 3 AVERAGES Calculations: Average Average for Unknown # 1: _∑x_ = sum of BP (ºC) n number of trials LIST OF KNOWNS n-butanol 2-propanol Ethanol Octane Ethyl acetate 1-octyl alcohol Density 0.809 0.786 0.789 0.703 0.773 0.8240 B.P. 117 ºC 82 ºC 78.4 ºC 126 ºC 78.9 ºC 194 ºC DATA TABLE# 3: Lab data comparing the AVERAGED DENSITY (g/mL), SLOPES (g/mL) (from graph), and BOILING POINTS with TRUE values for the unknowns. Unknown ID True Density (g/mL) Slope Density (g/mL) % Error slope Density Measured Averaged Density (g/mL) %Error Average Density (g/mL True BP (ºC) Measured Average BP (ºC) % Error BP (ºC) ERROR CALCULATIONS below data table: Calculate the percent error for your measurements. Be sure to provide the formula used for percent error. Show all work. Calculate the % error for your average Density, the slopes, and the BP. % Error = |true density (g/mL) - measured density (g/mL)|_ True density (g/mL x 100 % Error = |true BP (ºC) - measured BP (ºC) |_ x 100 True BP (ºC) DATA TABLE #4: Comparison of the Calculated average density; slope; and true value for each of the unknowns Percent Error (%) Unknown # 1 % Error Averaged Density Slope Boiling Point Bar Graph #1: Comparison of the Percent Error for each of the unknowns (should include your measured averages, slopes, and BP. CLASS ANALYSIS: Calculation for your group average DATA TABLE #5: Data table of class group averages for Density (g/mL) and Boiling Points (for Unknown) Group Number 1. 2. 3 4 5 6 7 8 9 10 11 12 13 14 15 Class range ∑x N (number of groups) X (Average/mean) Density (g/mL) Unknown #1 B.P (ºC). Unknown #1 DATA TABLE #6: CLASS PERCENT ERROR for Density and B.P. for 1 unknown liquid to the known values & Percent errors UNKNOWN Property Measured #1 Measured Value Known Value % Error Density (g/mL) Boiling Point (ºC) CALCULATIONS: % Error for Class averages for Density (g/mL) and Boiling Point (ºC) for Unknown #1 % Error = |true density (g/mL) - measured density (g/mL)|_ x 100 True density (g/mL % Error = |true BP (ºC) - measured BP (ºC) |_ x 100 True BP (ºC) BAR GRAPH #2: Bar Graph for Class % Error for Density and B.P. for Unknown liquid (also include your % error) CONCLUSIONS A. Was purpose met B. comments about percent error for density and boiling point (personal error) C. compare error for personal measurements and slope (for density) D. comment on class error E. compare personal error to class error F. discuss possible causes for the error (both personal and class) G. what needs to be done to decrease error (both personal and class) H. summary statement