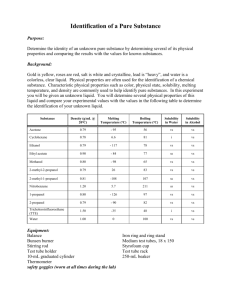
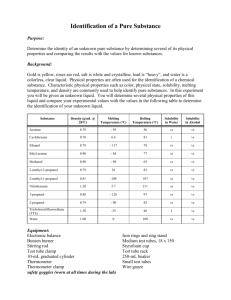
Observations of Substances
advertisement

Intro to Chemistry Chem1020 Lab Identification of an Unknown Liquid Chemistry Department Minneapolis Community & Technical College 1 Overview • Part I Introduction • Part II Solubility determination • Part III Density measurement • Part IV Boiling point determination • Part V Identification 2 Part I. Introduction In this experiment, you will be given a pure unknown liquid and the goal is to determine which one of the eight known substances this mysterious liquid is most likely to be. In order, you will determine its three physical properties (meaning that you are not changing its chemical composition throughout the experiment). They are: • solubility in water and ethyl alcohol, • density, and • boiling point. Make sure to record Then you will compare these properties with the properties of those of eight candidates. The best match will suggest the identify of your unknown liquid. this unknown number on your data sheet. Caution: All procedures must be performed in the fume hood. Make sure to cover the containers whenever they are moved out of the fume hood. 3 Part II. Solubility 1. Your instructor will first demonstrate that when two liquids are mixed, how will it look like when they are “soluble” or “insoluble”. 2. Then you will determine the solubilities of your unknown liquid in water and ethyl alcohol, respectively. Test tube 1: Test tube 2: 20 drops of unknown + 20 drops of water 20 drops of unknown + 20 drops of ethyl alcohol Soluble or Insoluble Soluble or Insoluble 4 Precautions!!! 1. Use the medicine dropper on your bench for the unknown liquid only. 2. Do not let the tip of any dropper touch the test tube, especially the inside. Put the dropper back to its original container after each use. 3. Before recording the observation, mix the two liquids by gently flicking the bottom of the test tube. 4. Dispose the wastes from both tubes into the designated waste container. 5 Part III. Density • You will use the same method as you did in the previous “Measurement” lab to determine the density of this unknown liquid. • Instead of a volumetric pipette, a 10-mL graduated cylinder will be used to accurately determine the volume of ~5 mL liquid. • Record the mass measurements as XX.XX g. • Record the volume measurements as X.XX mL. 6 1. 2. 3. 1. Determine and record the mass of an empty 10-mL graduated cylinder. 2. Use the medicine dropper to transfer approximately 5 mL of liquid into the cylinder. 5. 4. 3. Determine and record the volume of the liquid. (Often being forgotten) 4. Determine and record the total mass of cylinder and the liquid. 5. Pour the liquid into the large test tube on your bench for later boiling point measurement. 6. Repeat steps 2-5 for a second trial. 7 Sample Calculation of Density Trial 1 Trial 2 Vunknown 5.12 mL 4.99 mL Mcylinder +unknown 40.71 g 40.56 g Mcylinder 36.66 g 36.66 g Munknown = Mcylinder + unknown - Mcylinder 40.71g – 36.66g = 4.05 g (2 decimal places) 40.56g – 36.66g = 3.90 g (2 decimal places) 3.90g / 4.99mL Dunknown = Munknown / Vunknown 4.05g / 5.12mL = 0.791 g/mL = 0.782 g/mL Daverage = (D1 + D2 )/2 = (0.791 (3 + sig. 0.782)/2 = 0.787 g/mL fig.) (3 sig. fig.) • Since the difference between these two density determinations (0.791 – 0.782) = 0.009 g/mL is small enough (< 0.03 g/mL), a third trial is not necessary. • Follow the rules of significant figure calculation strictly. 8 Part IV. Boiling Point 1. Drop a piece of white rock, called boiling chip, into the large test tube. The chip ensures a smooth boiling and prevents “bumping”. 3. Lower the test tube into a water bath sitting on a hot plate. Make sure all the unknown liquid is submerged in the water bath. 1 cm 2. Insert a split-hole stopper with a thermometer into the test tube which should have ~10 mL of the unknown liquid. 4. Turn the knob on the right to dial “6” and start heating. 9 Part IV. Boiling Point • Watch the unknown liquid inside the large test tube closely. When there are abundant strings of bubbles arise from the boiling chip, read the thermometer immediately. Record this temperature as the boiling point in the form of XX.X ºC. Waiting too long will boil off too much liquid resulting in inaccurate determination. • Raise the large test tube so that it is out of the water bath. (Often forgotten) • Once cooled, dispose of the remaining unknown liquid and the boiling chip into the designated waste container. 10 Part V. Identification 1. Fill in the table on p.4 of the protocol with data of your unknown liquid. See the example shown in blue. 0.787 68.1 soluble soluble 2. First cross out unlikely candidates based on solubility. 3. The remaining four candidates unfortunately all have density around 0.79 g/mL, so we have to look for the one with a boiling point closest to 68.1 ºC , that of our unknown. It looks like methyl alcohol has the closest one. We got a winner! Acetone has the second closest boiling point, so can be put down as the 2nd most likely candidate. If our unknown had a boiling point of 61.0 ºC, then methyl alcohol and acetone would be equally possible. No conclusive determination can be made. 11 • Put all of the used glassware into the designated bins for later cleaning. Put the remaining unknown liquid at a designated place. • Wipe your station with wet sponge. • Please restock your station with clean glassware. Used small test tubes and medicine droppers Used large test tubes and graduated cylinders Vials with remaining unknowns 12