Foul Water Lab
advertisement

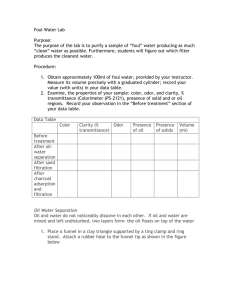
Foul Water Lab Name ____________________________________ Period ____________ Date ___________________ Introduction The purpose of this activity is to purify a sample of “foul” water, producing as much “clean” water as possible. Do not test the purity of the water by drinking it. Three water purification procedures will be used: (1) oil-water separation, (2) sand filtration, and (3) charcoal adsorption/filtration. (Filtration is a general term for separating solid particles from a liquid by passing the mixture through a material that retains the solid particles. The liquid collected after filtration is called the filtrate.) Procedure 1. Measure 100 mL of foul water with your graduated cylinder. 2. Examine the properties of your sample: color, odor, clarity, presence of solids on oily regions. Record your observations in the “Before treatment” section of your data table. Oil and Water Separation 1. Place the funnel with the attached rubber tubing in the funnel clamp. 2. Close the rubber tubing by using the pinch clamp. Shake or stir the foul water sample. Then pour half the sample in the funnel and let it stand for a few seconds until the two layers separate. 3. Carefully open the tube to release the lower layers into a 150-mL beaker. When the lower layer has drained out, quickly close the rubber tube. 4. Drain the remaining layer into a second 150-mL beaker. 5. Repeat steps 2-4 using the remainder of the sample, adding each liquid in the correct beaker. 6. Dispose of the top, oily layer as instructed by the teacher. Observe the properties of the remaining layer and measure its volume. Record your observations and data. Save the water sample for the next procedure. 7. Wash the funnel with soap and water. Sand Filtration 1. In foam cup with small whole at the bottom, place a premoistened layer of gravel and sand as shown in the picture. (The bottom gravel prevents the sand from washing through the holes. The top gravel keeps the sand from churning up when the sample is poured in.) 2. Gently pour the sample to be filtered into the cup. Catch the filtrate (filtered water) in a beaker as it drains through. 3. Dispose of the used sand and gravel according to your teacher’s directions. Do not pour sand or gravel into the sink! 4. Observe the properties and measure the volume of the water. Record your results. Save the water sample for the next procedure. Charcoal Adsorption/Filtration 1. Fold a piece of filter paper as seen in the picture. 2. Place the folder filter paper in the funnel. Wet the paper slightly so it adheres to the funnel cone. 3. Place the funnel in the funnel clamp. Lower the ring clamp so the funnel extends 2-3 cm inside a 150 mL beaker. 4. Place a small amount of charcoal into the funnel and pour the water sample slowly into the funnel. 5. If the filtrate is darkened by small charcoal particles, refilter the liquid. Use a clean piece of moistened filter paper. 6. When you are satisfied with the appearance and odor of your purified water sample, pour it into a graduated cylinder. Observe and record the properties and the final volume of the sample. 7. Return your purified water sample to your teacher. 8. Wash your hands thoroughly before leaving the laboratory. Foul Water Data and Questions Name ____________________________________ Date ____________________ Period __________ Data Table Color Clarity Odor Presence of Oil Presence of Solid Volume Before treatment After oil-water separation After charcoal adsorption/filtration Questions 1. Using the classification of matter presented in class, classify your initial sample of foul water. 2. What percent of the initial foul-water sample did you recover as “pure” water? Vol. water purified Vol. of foul water sample x 100% = percent of water purified ____________________ x 100% = % water purified 3. What volume of liquid did you lose during purification? 4. What percent of your initial foul-water sample was lost during purification? 5. During distillation, why did your teacher discard the first portion of the distillate? 6. Your teacher tested the electricity conductivity of your purified water sample. This test checks for the presence of dissolved, electrically-charged particles in the water. Record the results below. Sample Purified water sample Electricity Conductivity Tap water Distilled water What do these tests suggest about the purity of your water sample? 7. Distillation is not used by municipal water treatment plants. Explain.