SYLLABUS – Chapter 6 - Basic Science & Technology
advertisement

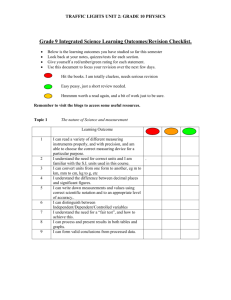
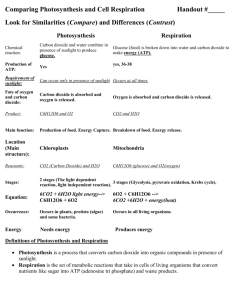
SYLLABUS – Chapter 6 Topic: Objectives: Students - 6. Air The low density and compressibility of air; the wind and resistance to rapid movement as examples of forces exerted by air; atmospheric pressure in relation to familiar examples/applications and the weather (descriptive treatment only). • explain and use correctly the words in italics below. • demonstrate awareness of the reality of air through simple practical activities. • explain the wind, and air resistance to rapid movement, as examples of forces exerted by air. • state that air has mass/weight, state the approximate density of air as 1 g/l and 1 kg/m3, and estimate the approximate mass of air in a given space. • state that air is compressible and demonstrate this through simple practical activities. • explain atmospheric pressure/air pressure with reference to the weight of the air in the atmosphere. • state that the atmosphere at sea level exerts a force of approximately 1 kg on every cm2. • state that air pressure acts equally in all directions. • state that the density of the air, and the atmospheric pressure, both decrease with height. • use air pressure to explain relevant everyday observations and applications. • state that wet, stormy weather is associated with low atmospheric pressure, that dry, calm weather is associated with high atmospheric pressure, and that winds blow from areas of high to areas of low atmospheric pressure. • correctly interpret simple observations involving combustion in a confined space. • correctly tabulate the approximate percentage composition of dry air (as given in Module 6-3). • describe the laboratory preparation of oxygen, including collection over water, sketch a suitable apparatus and write a simplified equation for the preparation in words. • identify oxygen in a test tube by carrying out the glowing splint test. • state how oxygen and nitrogen are manufactured and give a simple explanation of the process. • list the main properties and uses of oxygen (as given in Modules 6-4 and 6-6). • describe the laboratory preparation of carbon dioxide, including collection over water and by displacement of air, sketch a suitable apparatus and write a simplified equation for the preparation in words. • state how carbon dioxide is manufactured. • list the main properties and uses of carbon dioxide (as given in Modules 6-5 and 6-6). • list the main properties and uses of nitrogen and the inert gases (as given in Module 6-7). • explain the role of oxygen when things burn in air and write a simple word equation for combustion. • explain the fire triangle. • describe several simple, practical ways of putting out small fires and explain how they work. • describe simple examples of the following types of fire extinguishers: fire blanket, compressed carbon dioxide extinguisher, carbon tetrachloride extinguisher, dry powder extinguisher. • explain how each of these extinguishers works by isolating the fuel from heat or oxygen or both. • explain the role of oxygen when metals corrode in air and write a simple word equation for the rusting of iron. • identify water as a factor necessary for rusting, and salt as a factor that accelerates rusting. The composition of air; the laboratory preparation, simple properties, manufacture and main uses of oxygen and carbon dioxide (simple word equations to be introduced at this point); tests for oxygen and carbon dioxide; the simple properties, manufacture and main uses of nitrogen and the inert gases; The role of heat and oxygen in combustion, summary of the process of combustion in a simple word equation; flammable and non-flammable materials; the "fire triangle" and extinguishing fires. The role of atmospheric oxygen and water in the corrosion of metals with particular reference to rusting; salt as a factor which accelerates rusting; protection of iron and steel against rusting. 8 Topic: 6. Air (continued): The air and living things, differences in composition between inhaled and exhaled air, respiration, photosynthesis, the oxygen and carbon cycles. Objectives: Students • are aware that all living things need oxygen in order to produce energy by respiration and that carbon dioxide is a biproduct of this process. • write a simple word equation for respiration. • describe, and interpret the results of, simple experiments to show differences between inhaled and exhaled air. • list and explain four significant differences between inhaled and exhaled air. • are aware that green plants produce their own food, in the form of starch, by a process called photosynthesis; • are aware that photosynthesis uses energy from sunlight to convert carbon dioxide and water into starch with oxygen as a bi-product. • are aware that chlorophyll is necessary for photosynthesis. • state that chlorophyll is found in green bodies called chloroplasts which are present in many plant cells. • write a simple word equation for photosynthesis. • describe, and interpret the results of, simple experiments to demonstrate selected aspects of photosynthesis. • critically compare the word equations for combustion, respiration and photosynthesis. • explain how oxygen is recycled in nature and draw a diagram of the oxygen cycle. • explain how carbon is recycled in nature and draw a diagram of the carbon cycle. 9