CHE331.Lab#1.UV
advertisement
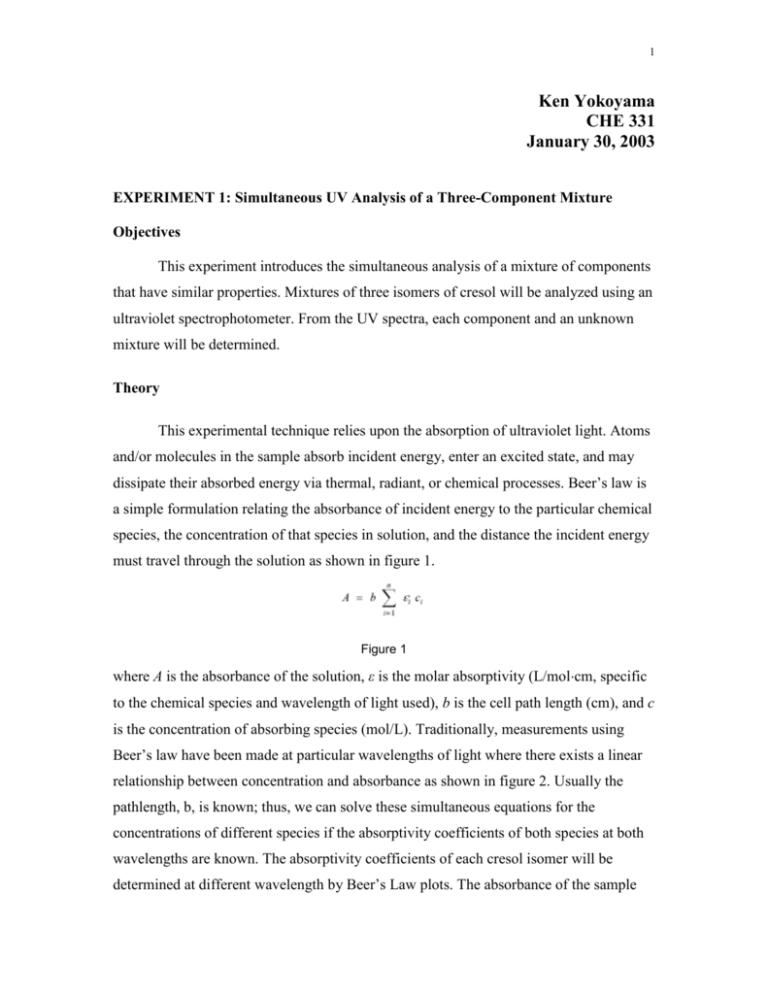
1 Ken Yokoyama CHE 331 January 30, 2003 EXPERIMENT 1: Simultaneous UV Analysis of a Three-Component Mixture Objectives This experiment introduces the simultaneous analysis of a mixture of components that have similar properties. Mixtures of three isomers of cresol will be analyzed using an ultraviolet spectrophotometer. From the UV spectra, each component and an unknown mixture will be determined. Theory This experimental technique relies upon the absorption of ultraviolet light. Atoms and/or molecules in the sample absorb incident energy, enter an excited state, and may dissipate their absorbed energy via thermal, radiant, or chemical processes. Beer’s law is a simple formulation relating the absorbance of incident energy to the particular chemical species, the concentration of that species in solution, and the distance the incident energy must travel through the solution as shown in figure 1. Figure 1 where A is the absorbance of the solution, ε is the molar absorptivity (L/molcm, specific to the chemical species and wavelength of light used), b is the cell path length (cm), and c is the concentration of absorbing species (mol/L). Traditionally, measurements using Beer’s law have been made at particular wavelengths of light where there exists a linear relationship between concentration and absorbance as shown in figure 2. Usually the pathlength, b, is known; thus, we can solve these simultaneous equations for the concentrations of different species if the absorptivity coefficients of both species at both wavelengths are known. The absorptivity coefficients of each cresol isomer will be determined at different wavelength by Beer’s Law plots. The absorbance of the sample 2 mixture will then be obtained for the same wavelengths; solution of simultaneous equations will thus enable us to obtain estimates of the cresol concentrations. Procedure I. First, determine the suitable wavelengths for the analysis by obtaining an absorption spectrum for m-cresol, p-cresol, and o-cresol separately. A. Prepare the following solutions: 1. Pipet 5, 10, 15 and 20 mL of the 0.05 g/L stock solution of p-cresol into a 25-mL volumetric flask and diluting to the mark. Mix well. 2. Repeat for m-cresol and o-cresol. 3. Follow the SOP for operation of the UVspectrophotometer. 4. Obtain the spectra for both solutions from 240 nm to 300 nm (or as narrow a range as possible). 5. Generate a Beer's Law (A vs. C) for all species II. Next, prepare mixtures of the following solutions: Mixtures #1 p-15 #2 m-15 #3 o-15 III. o-cresol 5 ml 5 ml 15 ml m-cresol 5 ml 15 ml 5 ml p-cresol 15 ml 5 ml 5 ml Finally, obtain unknown(s) from the instructor. This solution contains a mixture of the cresol isomers. Obtain its absorption spectrum, recording the absorbance at the wavelengths of interest. 3 Data 4 5 6 7 8 Results o-Cresol Conc.(mg/L) 10 20 30 40 50 1.2 Conc.(M) 0.0000924 0.000185 0.000277 0.000370 0.000462 Absorbance 0.16 0.32 0.5 0.76 0.82 y = 2391.2x + 0.005 R2 = 0.9994 p-Cresol Standard Curve Absorbance 1 0.8 0.6 0.4 0.2 0 0.0000 0.0001 0.0002 0.0003 0.0004 Concentration (M) 0.0005 9 m-Cresol Conc.(mg/L) 10 20 30 40 50 1.2 Conc.(M) 0.0000924 0.000185 0.000277 0.000370 0.000462 Absorbance 0.2 0.39 0.59 0.78 0.97 y = 2088.3x + 0.007 m-Cresol Standard Curve R2 = 0.9999 Absorbance 1 0.8 0.6 0.4 0.2 0 0.0000 0.0001 0.0002 0.0003 0.0004 Concentration (M) 0.0005 10 p-Cresol Conc.(mg/L) 10 20 30 40 50 1.2 Conc.(M) 0.0000924 0.000185 0.000277 0.000370 0.000462 Absorbance 0.22 0.45 0.67 0.9 1.1 y = 2391.2x + 0.005 R2 = 0.9994 p-Cresol Standard Curve Absorbance 1 0.8 0.6 0.4 0.2 0 0.0000 0.0001 0.0002 0.0003 0.0004 Concentration (M) Isomer Molar Absorptivity Constant o-Cresol 1904.3 M m-Cresol 2088.3 M p-Cresol 2391.2 M 0.0005






