ChE 471 Exam 3 Fall 2006
advertisement
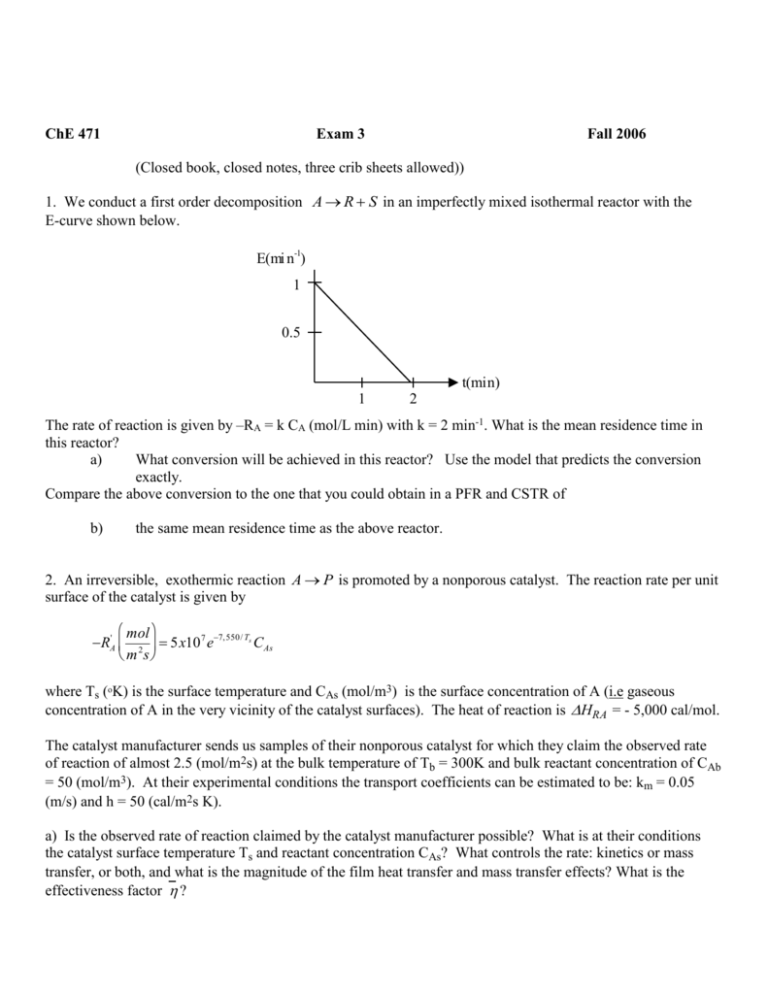
ChE 471 Exam 3 Fall 2006 (Closed book, closed notes, three crib sheets allowed)) 1. We conduct a first order decomposition A R S in an imperfectly mixed isothermal reactor with the E-curve shown below. -1 E(mi n ) 1 0.5 1 2 t(min) The rate of reaction is given by –RA = k CA (mol/L min) with k = 2 min-1. What is the mean residence time in this reactor? a) What conversion will be achieved in this reactor? Use the model that predicts the conversion exactly. Compare the above conversion to the one that you could obtain in a PFR and CSTR of b) the same mean residence time as the above reactor. 2. An irreversible, exothermic reaction A P is promoted by a nonporous catalyst. The reaction rate per unit surface of the catalyst is given by mol RA' 2 5x10 7 e7, 550/ Ts CAs m s where Ts (oK) is the surface temperature and CAs (mol/m3) is the surface concentration of A (i.e gaseous concentration of A in the very vicinity of the catalyst surfaces). The heat of reaction is HRA = - 5,000 cal/mol. The catalyst manufacturer sends us samples of their nonporous catalyst for which they claim the observed rate of reaction of almost 2.5 (mol/m2s) at the bulk temperature of Tb = 300K and bulk reactant concentration of CAb = 50 (mol/m3). At their experimental conditions the transport coefficients can be estimated to be: km = 0.05 (m/s) and h = 50 (cal/m2s K). a) Is the observed rate of reaction claimed by the catalyst manufacturer possible? What is at their conditions the catalyst surface temperature Ts and reactant concentration CAs? What controls the rate: kinetics or mass transfer, or both, and what is the magnitude of the film heat transfer and mass transfer effects? What is the effectiveness factor ? b) We want to reproduce these findings and run. the same type of experimental reactor at identical velocity conditions and at identical bulk temperature and concentration reported above conditions. However, we observe a very low rate of 0.040 (mol/m2s). Explain and try to quantify these observations. What surface temperatures and concentrations correspond to the rate observed by us? What are the magnitudes of the film effects and what is the effectiveness factor at our observed rate? What conclusions do you reach? Can both results be right? 3. What can you tell about the controlling resistance (film, diffusion, kinetic) and activation energy for a 1st order catalytic reaction on porous catalyst particles based on the data below obtained in a stirred basket reactor that exhibits CSTR behavior. Isothermal conditions prevail in the reactor during each run. Run1 Flow Rate Feed Conc. Outlet Conc. Cat. Mass Cat.Diam. RPM m 3 mol mol W g d p cm Q CAo 3 CA 3 m m h Tb C 1 0.05 50 10 10 1 low 340 2 0.02 40 10 6 2 high 340 3 0.08 20 10 4 2 high 390 4 0.09 30 10 9 2 low 390 The density of the catalyst pellets is p 2.5 g cm 3 . The heat of reaction is rA 5,000cal mol A, the heat transfer coefficient to the catalyst pellets is h 1000 cal m 2 sK , mass transfer coefficient is about 1(m/s), catalyst particles are spherical, the effective diffusivity in them is Deff 0.01 cm 2 s and the effective conductivity is eff 0.12cal msK . [PAY ATTENTION TO UNITS AND CONVERT THEM TO A CONSISTENT SET AS YOU HAVE HOURS AND SECONDS AND METERS AND CENTIMETERS ETC. FIRST NOTE THAT YOU NEED THE RATE PER UNIT VOLUME OF CATALYST PELLET NOT PER UNIT MASS]. Your goal is to find the dominant resistance (film, pore, kinetic) and fully explain your answer. a. b. c. Can the above large catalyst pellets be considered internally isothermal? What is the maximum pellet? Is it important? What can we say about the external mass transfer effects based on the results in the above table? Is that finding confirm by your analysis of the film effect based on the observed rate and mass transfer coefficient.? What can we say about the film heat transfer effects? Is the particle surface temperature about the same as in the bulk? What is Ts ? d. e. What can you say about internal diffusion effects? Based on all of the above can you estimate the activation energy for this reaction? How about the catalyst particle effectiveness factor value at the two temperatures? 4. Sketch the corresponding xA vs T diagram for the two packed bed reactors operated adiabatically for an exothermic reaction as per flow chart shown below. A B M N C Data: consistent. D E P Q F x AA 0, x A N 0.6, x A Q 0.9 R1 = 2 R2 = 1 Both heat exchangers are cooling the flow. Assume a slope of the adiabatic line and be G