Packed Bed - Chemical Engineering
advertisement

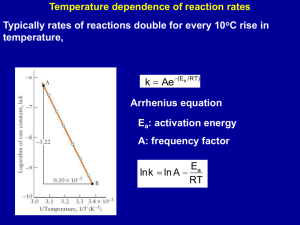
Pressure drop in Packed Bed Reactors Chemical Reaction Engineering I Aug 2011 - Dec 2011 Dept. Chem. Engg., IIT-Madras Overview • Notation • PFR design equation (mass balance) • Pressure drop equation for packed bed • • • • Modified for catalytic reactions Accounts for change in number of moles (due to reaction) Does not consider phase change Methodology, with examples Basics • Usual notation applies. • Reaction is usually written as rA , in mol/vol/time • For catalytic reactions, it is written as • rA’, in mol/g-catalyst/time • Rate law is written as rA' kCAn • Where the rate constant k is in appropriate units • Remember that CA is in mol/vol • Packed bed: The PFR equation will be modified. Instead of dV, we will use dW dFA rA' dW Design Equation For constant temperature and with ideal gas law FAin F dx k A dW Q n F 1 x k n Ain Pn PinnQinn 1 x n 1 dx kFAin n n dW Pin Qin n n 1 x P n 1 x n Pressure Drop Equation Ergun equation, written in terms of mass flow rate dP G 1 150 1 1.75G 3 dz Dp Dp Need not memorize this Superficial mass velocity G = Vsup Porosity Diameter of particles is Dp is a variable. It should be written in terms of X and P Similarly, z must be written in terms of W Pressure drop equation • Mass flow rate is a constant Q inQin inQin Q • For ideal gas, constant temperature, no phase change inQin P in P PinQin 1 x Pin 1 x Pin 1 x dP G 1 150 1 1.75G 3 dz D p Dp in P Pressure drop equation • Relate ‘W’ to ‘z’ • Weight of catalyst = Volume of catalyst * density of catalyst • W = Az * (1-) * c. • dW = A (1-) * c * dz • For ideal gas, constant temperature, no phase change Pin 1 x dP 1 G 1 150 1 1.75G 3 dW A1 c Dp Dp in P 1 x dP dW P Simplifications • If there is no change in volume due to reaction (if = 0) • Pressure equation can be solved P2 P02 2W • If parameter is small, and X is also small, then we can neglect that in the pressure drop equation, but not in the design equation Examples Pin = 5e5; %Pa; T = 400 ; %Kelvin Qin = 0.01 ; % m3/s M = 50 ; % molecular weight, g/gmol dia = 0.1; % diameter in m mu = 2e-5; % viscosity in Pa-s k = 0.001; % rate constant with correct units. lit/g-catalyst/s, for example n = 1; %order of reaction epsilon = -0.2; % fractional change in volume for 100% conversion , for the given feed phi = 0.3 ; % Void fraction, volume of void/total volume RhoC = 2000 * 1000; % catalyst density, in g/m^3 W_end = 1000; % in g Dp = 0.005; % diameter of particle, in m Solution Rho in – 7.5174 kg/m3 Fain = 1.5035 gmol/s Area = 0.0079 m2 Va-in=1.2732 m/s G = 9.5715 kg/s Alpha = 5.155 E6, Pa2/g catalystt Beta = 1.6 e-9 units Results Conversion and Pressure vs Catalyst Weight Conversion 0.8 0.6 0.4 d = 10 cm = -0.2 0.2 0 0 100 200 300 400 500 600 700 800 900 1000 400 500 600 700 800 900 1000 Pressure / Pa 5 5 x 10 4.95 d = 10 cm = -0.2 4.9 0 100 200 300 Catalyst Weight / g Solution If dia is changed from 10 cm to 5 cm Conversion and Pressure vs Catalyst Weight Conversion 0.06 0.04 d = 5 cm = -0.2 0.02 0 0 50 100 150 200 250 300 350 400 150 200 250 300 350 400 5 Pressure / Pa • 6 x 10 4 d = 5 cm = -0.2 2 0 0 50 100 Catalyst Weight / g