Lect 13 Mole & Molar Mass
advertisement

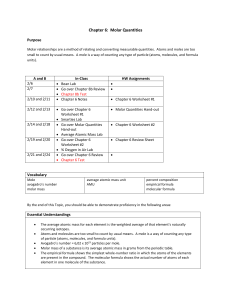
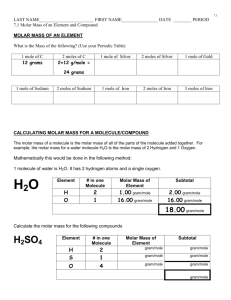
MOLE & Avogadro Number No way to count atoms one by one. However, we need to know what amount of each reagent to take in order the rxn to proceed completely. Example: Mg + S MgS How much of Mg & S to take in other they react completely, with no unreacted material? From the equation: 1 atom Mg (i.e. 24 a.m.u.) combines with 1 atom of S (i.e. 32 a.m.u.). Mass ratio, 24:32, does not depend on units: it will be the same in a.m.u. in any other units, say, in gram. Take 12 g Mg & 32 g S: they will react completely. 12 g is the molar mass of Mg 32 g the molar mass of S. i.e. 12 g Mg react w/ 32 g S. Two units of mass: atomic mass unit & gram: how are they related to each other? 1 g = 6.02.1023 a.m.u. in H2 + F2 2HF a.m.u.: 2 38 40 g: 2 38 40 2 g of H2 gas react with 38 g F2 gas producing 40 g HF gas Since they react 1:1 on atomic basis, they must contain the same number of atoms, exactly the same, that relates gram to a.m.u. This number is Avogadro number, NAv = 6.02.1023 This number of particles is called a MOLE. Mole is a counting unit (like dozen, gross, etc.) The equation: Mg + S MgS tells us not only that Mg & S combine 1 atom:1 atom, but also that they combine 1 mole Mg to 1 mole S. Molar mass is the mass of 1 mole. Molar mass of an element in grams is numerically equal to the atomic mass of the element (in a.m.u.) Molar mass of an ionic compound is the sum of molar masses of constituent elements in its FORMULA UNIT. Molar mass of a molecular compound is the sum of molar masses of constituent elements in its molecule.