Chemistry-notes-ch-11
advertisement

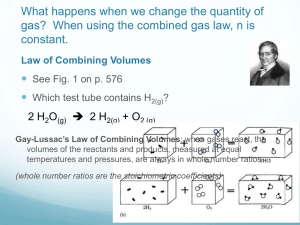
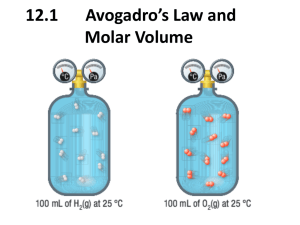
Chemistry Notes: Chapter 11 Volume/Mass Relationships of Gases Gay-Lussac’s law of combining volumes of gases at constant temperature and pressure: the volumes of gaseous reactants and products can be expressed as ratios of small whole numbers. Ex. 2 volumes of H2 combines with 1 volume of O2 to yield 2 volumes of water vapor. Avogadro’s Law—equal volumes of gases a the same temperature and pressure contain equal numbers of molecules. Avogadro also discovered that some atoms are diatomic. This law indicated that gas volume is directly proportional to the amount of gas, at a given temp. and pressure. V = kn or V/n = k where n is the amount of gas in moles and k is a constant. Standard Molar Volume of a gas—the volume occupied by one mole of gas at STP. One mole of any gas at STP occupies 22.4 L. This is the standard molar volume for any gas. Consequently, 1 mol/22.4 L can be used for conversions (sample problem 11-1. 11-2 Ideal Gas Law—Derived from Boyle’s Law, Charles’s Law, Gay-Lussac’s Law and Avogadro’s Law: It is a mathematical relationship among pressure, volume, temperature, and the number of moles of a gas. P. 341 V = nRT/P or PV=nRT where P is pressure in atm., V is volume in Liters, n is the number of moles, T is temperature in Kelvin and R is the gas constant. R = .08205784 Latm/mol K To use this equation, solve for what variable you are looking for, make sure your known quantities are in the proper units, fill in what you know and solve the equation. Make sure your answer has the proper units. P. 343-344. An equation for finding the density of a gas can be derived from the Ideal Gas Law. It is D = MP/RT where M is the molar mass, P is pressure, R is the gas constant and T is the temperature. Sample problem 11-6 11-3 Stoichiometry Volume-Volume Calculations: Start with what you know and then multiply by the ratio from the balanced equation. (sample problem 17) Volume-Mass or Mass-Volume calculations The ideal gas law can be useful for calculating values at standard and nonstandard conditions. (sample problems 11-8 and 11-9) However, if all reactants and products are at STP, the factor label method may be used with the 22.4 L/mole ratio. 11-4 Graham’s Law of Effusion—rates of effusion of gases at the same temperature and pressure are inversely proportional to the square roots of their molar masses. (Go over p. 351 and 352) Rate of effusion of A = √Mb where m is the molar mass Rate of effusion of B √Ma 1