The Atomic Mass of Candium
advertisement

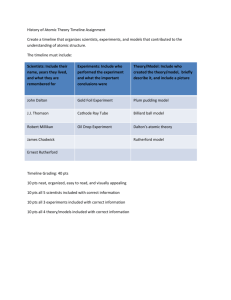
Name: ______________________________________ Date: ________________ Period: ______ The Atomic Mass of Candium Purpose: To analyze the isotopes of “Candium” and to calculate its atomic mass. Materials: Sample of Candium Balance Procedure: (30 pts) Obtain a random sample of “Candium” by filling a 150mL beaker. Calculate your sample’s average atomic mass. Make certain your balance is set to “grams” and round all answers to 2 decimal places. Record your data neatly and clearly in a table. Show all work and formulas. Use the lines below to outline how you will calculate your sample’s average atomic mass. Your teacher must sign your calculation procedures before you may get your Candium sample. ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Sample #1 Data Table: (15 pts) Sample #1 Average Atomic Mass: _______________________________ (10 pts) Analysis: (10 pts) 1. Explain any differences between the atomic mass of your Candium sample and that of your neighbor. Explain why the difference would be smaller if larger samples were used. ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ 2. Determine the atomic mass of a second sample of Candium. How does it compare with your first? Suggest reasons for any differences between the samples. ___________________________________________________________________________ ___________________________________________________________________________ Sample #2 Data Table: (15 pts) Sample #2 Average Atomic Mass: _______________________________ (10 pts) More Analysis (10 points): 3. Why are the atomic masses for most elements not whole numbers? 4. How are the three isotopes of hydrogen (hydrogen-1, hydrogen-2, and hydrogen-3) alike? How are they different? Answer in terms of subatomic particles.