Atomic Mass Unit
advertisement

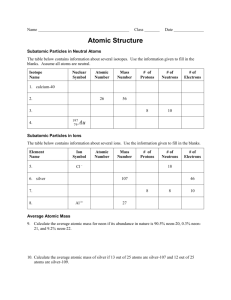
Atomic mass and formula Average atomic mass The weighted average of the masses of the isotopes of an element. Molecular Formula is a chemical formula of a molecular compound that shows the kinds and number of atoms present in a molecule of a compound. (H2O) Molar mass A term used to refer to the mass of a mole of any substance. C =12 grams Atomic Mass Unit A unit of mass equal to 1/12 the mass of an atom Of the most common isotope of carton (carbon 12),which is assigned a mass of 12. Examples C= 12 grams. AMU is = to 1/12 of a carbon atom or Hydrogen=l gram Isotope- One of two or more species of atoms of a chemical element having nuclei with the same number of protons but different numbers of neutrons. They have the same atomic number of protons but different numbers of neutrons. They have the same atomic number and hence nearly identical chemical behavior but different atomic masses. Most elements found in nature are mixtures of several isotopes Carbon-14, for instance, is used for estimating the age of objects within a relatively recent span of time-up to about 5,000 years-where as geologists and other scientists use uranium-238 to date minerals of an age on a scale with that of the Earth.