Authors: E

Authors: E. Hoekstra, J.A. Hogendoorn, S.R.A. Kersten, W.P.M. van Swaaij
Adress: University of Twente, The Netherlands, TNW-TCCB
Fast pyrolysis is a process in which organic materials are rapidly heated to 450 - 500 °C in absence of oxygen. Under these conditions, organic vapors, gases and char are formed. The vapors are condensed to pyrolysis oil. Pyrolysis products are formed by primary reactions followed by secondary reactions which results in a mixture of over 300 oxygenated organic components. A variety of reactor configurations has been investigated and developed
1 among which (circulating) fluidized bed -, entrained flow – ablative -, rotating cone -, auger
2
– and vacuum pyrolysis reactors. Different vapour residence time – temperature profiles arise in these reactor types. Secondary cracking and polymerization reactions are known to occur in the vapour phase at high temperatures
3
. The goal of this research is to identify the pyrolysis products formed during the initial stage of the pyrolysis process. By knowing the primary fast pyrolysis process it might be possible to identify possibilities to improve the oil quality.
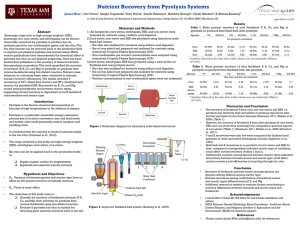
To achieve this goal, a novel wire-mesh reactor was developed and constructed at the
University of Twente. In a wire mesh reactor, a finely ground biomass sample is sandwiched between two wire mesh layers, which are heated directly by an electrical current till a pre-set temperature. The mesh retains both the wood and char particles, but offers little resistance to the passage of vapours and gases. In our concept, the wire mesh is placed in a vacuum box immersed in liquid nitrogen (-195
0
C). So, negligible secondary reactions will occur, because the freshly formed vapours and gases leave the hot wire mesh very rapidly and will condense immediately on the wall.
In this study, results obtained in the wire-mesh reactor are compared with these obtained in a more conventional fluidized bed reactor (both using the same pine wood feed)
4
. Significantly higher oil and lower char and gas yields were observed in the wire mesh-reactor. The primary pyrolysis oil has a higher molecular weight (SEC), contains less aliphatic structures (NMR) and probably more sugars (extraction and HPLC) than pyrolysis oil originating from the fluidized bed reactor.
Furthermore, the influence of temperature, pressure and hold-time was studied in the wiremesh reactor in more detail. The yields, gas composition, pyrolysis oil quality (SEC/NMR), char structure (SEM) and vapour deposition rates (high speed camera) were compared with each other. The results show both a significant influence of temperature and pressure and a less significant effect of hold time.
1 A.V. Bridgwater, G.V.C. Peacocke, Fast pyrolysis processes for biomass. Renewable Sustainable Energy ReV.
2000, 4 (1), 1–73.
2 L. Ingram, D. Mohan, M. Bricka, P. Steele, D. Strobel, D. Crocker, B. Mitchell, J. Mohammed, K. Cantrell,
C.U. Pittman, Pyrolysis of wood and bark in an auger reactor: physical properties and chemical analysis of the produced bio-oils, Energy and Fuels, 2008, 22, 614-625
3 S.R.A. Kersten, X. Wang, W. Prins, W.P.M. van Swaaij, Biomass pyrolysis in a fluidized bed reactor. Part 1: literature review and model simulations, Ind. Eng. Chem. Res., 2005, 44, 8773-8785
4 E. Hoekstra, K. J. A. Hogendoorn, X. Wang, R. J. M. Westerhof, S. R. A. Kersten, W. P. M. van Swaaij, and
M. J. Groeneveld, Ind. Eng. Chem. Res. 2009, 48, 4744–4756