Experiment Synthesis of Butyl Acetate
advertisement

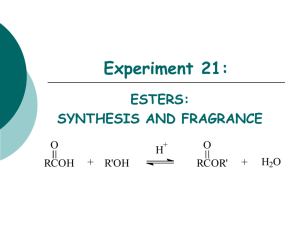
Experiment Synthesis of Butyl Acetate 1. Aim of experiment (1) Understood the basic synthesis principle and basic operation of organic acid ester under acid catalysis. (2)Master the basic operation of back flow dividing water, lavation, dryness and distillation. 2.Principle of experiment The main reaction: O O + H3C H3C OH H3C OH O CH3 The side eaction: 13 H3C 11 12 14 4 OH 10 H3C 5 2 3 6 O 8 7 1 CH3 9 3. Main apparatus and chemical reagents Distillation flask, bulb condensation pipe, distillation conk, frank prolong, tail pipe, three-mouth flask. N- butyl alcohol, ice vinegar, concentrated sulfuric acid, soda solution, anhydr-sal epsom, pH test paper. 4. Experiment procedures (1) Back flow dividing water. 14.4mL acetic acid and 28.9mL alcohol are put into the 250mL distillation flask. The 10 drop concentrated sulfuric acid is put into the 250mL distillation flask. Several zeolites are put into. Fit the bulb condensation pipe and dividing water organ on the 250mL distillation flask. Put through condensate water. Heat distillation flask until the liquid inside it refluxes about 40min. (2) Divide the liquid and clean it. Cool when back flow end. Take the bulb condensation pipe. The layer of ester from dividing water organ and reaction liquid from three-mouth flask are put into separating funnel together. Clean with 20mL water. Divide the layer of water. (3) Remove acetic acid. Drip 25mL soda solution slowly into three-mouth flask which can be gotten together distillation cut. The gas of CO2 is transgressed after rocking the three-mouth flask continually. Check up the upper layer inside three-mouth flask with litmus paper. Divide the layer of water when the upper layer isn’t red. (4)Dryness. The layer of ester is put into dry conical flask. The anhydr-sal Epsom is put into it. Desiccate it. (5) Distillation and purification. The Butyl Acetate after dryness is shift to a dry 50mL distillation flask. Fix distillation equipment with dry instrument. Several zeolites are put into. Distill and get together the distillation cut between 124℃ and 126℃. Record the boiling point of distillate in detail. Quantify the result and calculate rate of production. 5. Matters need attention (1) Tail water when the water refluxes into the reactive system. (2) When the reaction go on about 40min and no sweat sink in the dividing water organ, the reaction end. 6. Questions (1) How do we get rid of water in the reaction? (2) How to calculate the dividing water which can ensure reaction safe.