Chapter Seventeen Outline
advertisement

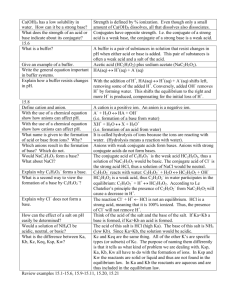
A.P. Chemistry Chapter 17 Outline: Buffers and Ksp I. Buffer Solutions: solutions that resist changes in pH when limited amounts of acid or base are added to it A. Buffering Action 1. A buffer must contain a weak acid that can react with the added base and a weak base that can react with the added acid 2. Buffers usually consist of approximately equal quantities of a weak acid and its conjugate base, or a weak base and its conjugate acid. B. The pH of Buffer Solutions 1. Can be calculated by using H3O+ in the Ka expression 2. The Henderson-Hasselbach Equation an be used to calculate pH and also to determine the ratio of conjugate base to conjugate acid concentrations needed to achieve buffers of a given pH Ph = pka + log [conjugate base/conjugate acid] This equation has two limitations: The ratio must be between 0.1 and 10, and the concentrations of both conjugates must exceed their ka values by a factor of at leas a 100. 3. A buffers pH equals the pka of its weak acid when the concentrations of the acid and base are equal 4. The ph range of a buffer is limited to about one pH unit above or below the pka of the conjugate acid C. Buffer Capacity: the quantity of acid or base the buffer can accommodate without undergoing significant pH changes II. III. Acid-Base Titrations A standard solution, one whose concentrations is known accurately, is added from a buret to an acid or base which concentration is to be determined. The solution in the buret is known as the titrant and the equivalence point is reached when stoichiometric amounts of titrant have been added to neutralize the unknown. The end point of a titration occurs when the indicator turns color. Titration curves are graphs of pH as a function of the volume of the titrant added. Titrations of a Polyprotic Acid with Base: polyprotic acids react stepwise when titrated with bases Acid Rain: the reaction of water with carbon dioxide, nitrogen dioxide, and sulfur oxides IV. Solubility Equilibria and the Solubility Product, ksp An equilibrium expression can be written to indicate the extent to which a solid solute dissolves to form ions The solubility is the amount of solute per unit volume of solution (mol/L) that dissolves to form a saturated solution. The solubility product constant is the equilibrium constant for the chemical equilibrium that exists between a solid ionic solute and its ions in a saturated solution. 2. 3. 4. 5. VI. Factors Affecting Solubility The effect of acids and pH The presence of common ions the formation of complex ions amphoterism VII. Will Precipitation Occur precipitation may nor occur if the concentrations are less than 0.1M if Q<ksp no ppt if Q>ksp ppt forms if Q=ksp the solution is saturated and is at equilibrium and at the point of precipitation