Name - Dorman High School
advertisement
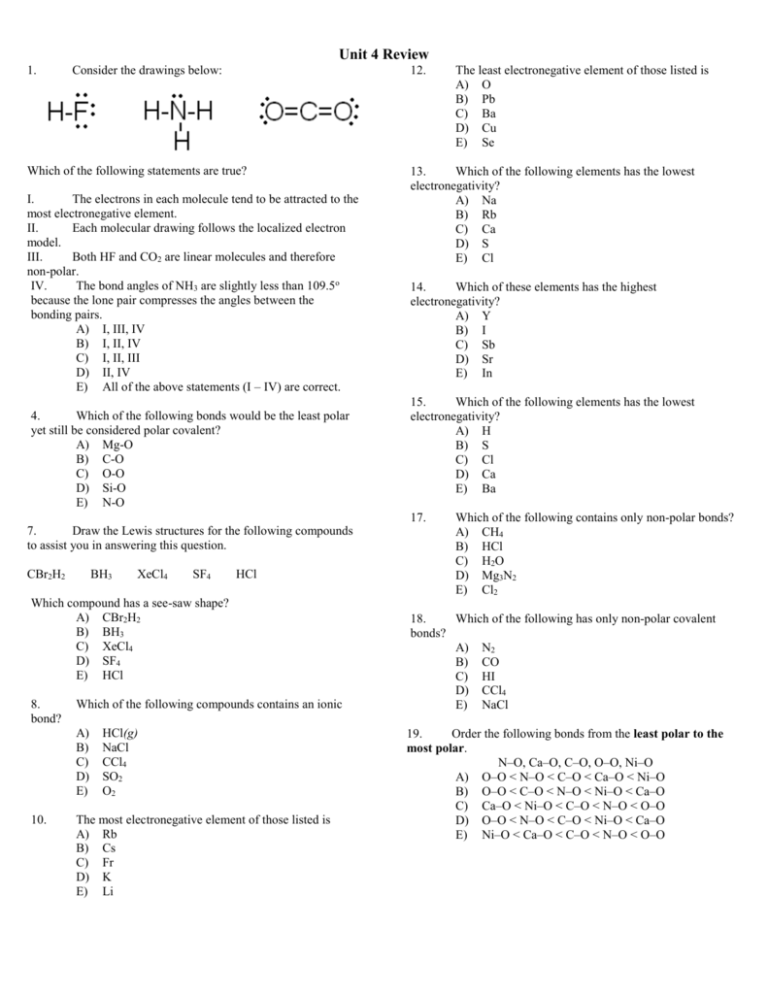
Unit 4 Review 1. Consider the drawings below: 12. Which of the following statements are true? I. The electrons in each molecule tend to be attracted to the most electronegative element. II. Each molecular drawing follows the localized electron model. III. Both HF and CO2 are linear molecules and therefore non-polar. IV. The bond angles of NH3 are slightly less than 109.5o because the lone pair compresses the angles between the bonding pairs. A) I, III, IV B) I, II, IV C) I, II, III D) II, IV E) All of the above statements (I – IV) are correct. 4. Which of the following bonds would be the least polar yet still be considered polar covalent? A) Mg-O B) C-O C) O-O D) Si-O E) N-O 13. Which of the following elements has the lowest electronegativity? A) Na B) Rb C) Ca D) S E) Cl 14. Which of these elements has the highest electronegativity? A) Y B) I C) Sb D) Sr E) In 15. Which of the following elements has the lowest electronegativity? A) H B) S C) Cl D) Ca E) Ba 17. 7. Draw the Lewis structures for the following compounds to assist you in answering this question. CBr2H2 BH3 XeCl4 SF4 HCl Which compound has a see-saw shape? A) CBr2H2 B) BH3 C) XeCl4 D) SF4 E) HCl 8. bond? Which of the following compounds contains an ionic A) B) C) D) E) 10. HCl(g) NaCl CCl4 SO2 O2 The most electronegative element of those listed is A) Rb B) Cs C) Fr D) K E) Li The least electronegative element of those listed is A) O B) Pb C) Ba D) Cu E) Se Which of the following contains only non-polar bonds? A) CH4 B) HCl C) H2O D) Mg3N2 E) Cl2 18. Which of the following has only non-polar covalent bonds? A) N2 B) CO C) HI D) CCl4 E) NaCl 19. Order the following bonds from the least polar to the most polar. N–O, Ca–O, C–O, O–O, Ni–O A) O–O < N–O < C–O < Ca–O < Ni–O B) O–O < C–O < N–O < Ni–O < Ca–O C) Ca–O < Ni–O < C–O < N–O < O–O D) O–O < N–O < C–O < Ni–O < Ca–O E) Ni–O < Ca–O < C–O < N–O < O–O Unit 4 Review 20. Which of the following atoms has the greatest electronegativity? A) Na B) Rb C) Cl D) Se 33. What is the number of formula units in 0.00300 moles of Sr(NO3)2? A. 5.96 X 10 23 B. 6.02 X 10 23 C. 3.00 X 10 21 D. 1.81 X 10 21 23. If atom X forms a diatomic molecule with itself, the bond is A) ionic B) polar covalent C) non-polar covalent D) polar coordinate covalent E) none of these 34. What is the percent composition of Br in LiBr? A. 7.99% B. 43.42% C. 6.94% D. 92.01% 27. When a molecule has a center of positive charge and a center of negative charge, it is said to have a ______________. A) magnetic attraction B) diatomic bond C) double bond D) polyatomic ion E) dipole moment 28. One of the most important characteristics of the water molecule is its ______________, which allows it to surround and attract both positive and negative ions. A) polarity B) strength C) magnetism D) fluidity E) stability 29. What is the percent composition of Li in LiBr? A. 7.99% B. 6.94% C. 43.42% D. 92.01% 30. What is the molecular formula of a compound that has an empirical formula of C2H4S, and an experimental molar mass of 179? A. C6H12S3 B. CHS C. C4H8S2 D. C2H4S 31. What is the percent of oxygen in HNO2? A. 2.15% B. 68.06% C. 14.01% D. 29.80% 32. What is the molecular formula for a compound that is 64.27% carbon, 7.19% hydrogen, and 28.54% oxygen, and an experimental molar mass of 168.19? A. C12H2O4 B. C9H12O3 C. C3H4O D. C6H8O2 35. What is the molecular formula for a compound that is 41.39% carbon, 3.47% hydrogen, and 55.14% oxygen, and an experimental molar mass of 116.07? A. C2H2O2 B. C6HO6 C. C4H3O5 D. C4H4O4 36. If a compound is 66.0% barium and 34.0% chlorine, what is the empirical formula? A. Ba2Cl B. Ba3Cl2 C. BaCl2 D. BaCl 37. What is the mass, in grams, of 3.00 moles of SeOBr 2? A. 764 g B. 467 g C. 84.92 g D. 254.76 g 38. What is the formula mass for C6H3Cl3? A. 581.64 B. 48.47 C. 181.44 D. 118.44 39. What is the molecular formula of a compound that has an empirical formula of C2H4O, and an experimental molar mass of 176? A. C4H8O2 B. C2H4O C. C8H16O4 D. C6H12O3 40. What is the percent of hydrogen in HNO 2? A. 68.06% B. 14.01% C. 2.15% D. 29.80% 41. What is the percent of silver in Ag2S? A. 10.87% B. 12.94% C. 87.059% D. 32.07% Unit 4 Review 42. What is the empirical formula for a compound that is 60.9% As and 39.1% S? A. AsS2 B. AsS C. As3S2 D. As2S3 43. What is the mass, in grams, of 488 moles of calcium carbonate? A. 4.88 X 10 4 g B. 6.02 X 10 23 g C. 4.88 X 10 4 g D. 488 X 10 4 g E. 160.1 g 44. What is the number of molecules in 0.989 moles of C6H5NO2? A. 6.95 X 10 23 B. 5.96 X 10 23 C. 9.89 X 10 23 D. 6.02 X 10 23 45. The mass of one mole of a compound is known as its A. metric mass B. unknown mass C. atomic mass D. molar mass 46. What is the percent composition of sulfur in Ag2S? A. 12.94% B. 87.059% C. 10.87% D. 32.07% 47. What is the formula mass for Al2S3? A. 194.25 B. 145.06 C. 118.08 D. 150.14 48. What is the molecular formula for a compound that is 54.53% carbon, 9.15% hydrogen, and 36.32% oxygen, and an experimental molar mass of 88? A. C5H2O3 B. C10H2O5 C. C4H8O2 D. C4HO2 49. What is the percentage of oxygen in NH4NO3? A. 5.05% B. 35.00% C. 59.96% D. 14.01% 50. What is the percentage of carbon in C14H10? A. 94.33% B. 12.01% C. 6.50% D. 5.67% 51. What is the number of formula units in 4.27 moles of WO3? A. 2.57 B. 4.27 C. 2.57 X 10 24 D. 4.7 X 10 23 52. What is the molecular formula of a compound that has an empirical formula of C2H2O, and an experimental molar mass of 254? A. C4H4O2 B. C12H12O6 C. C6H6O3 D. C2H2O 53. What is the mass, in grams, of 6.00 X 10-8 moles of nicotine (C10H14N2)? A. 6.02 X 10 23 g B. 9.74 X 10 -6 g C. 162.24 g D. 973.44 g 54. What is the emperical formula for a compound that is 76.89% Re and 23.12% O? A. ReO B. ReO4 C. Re2O7 D. Re7O2 55. In an ionic compound, the chemical formula is generally the same as the A. molecular formula B. molar mass C. empirical formula D. formula mass 56. What is the molecular formula of a compound that has an empirical formula of C2H3O2, and an experimental molar mass of 119? A. C6H9O6 B. C2H3O2 C. C4H6O4 D. C8H12O8 57. What is the formula mass for B4H10? A. 53.34 B. 46.14 C. 48.52 D. 57.61 58. A 0.858 g sample of an unknown substance is composed of 0.537 g of copper and 0.321 g of fluorine. What is the empirical formula? A. Cu3F2 B. Cu5F3 C. CuF2 D. Cu2F Unit 4 Review 59. What is the formula mass for Nb5O2? A. 556.94 B. 659.45 C. 412.08 D. 496.55 64. A 46.25 g sample contains 14.77 g of potasium, 9.06 g of oxygen, and 22.42 g of tin. What is the empirical formula? A. KSnO B. K4Sn2O6 C. K2SnO3 D. KSn2O3 60. A 0.0082 g sample contains 0.0015 g of nickel and 0.0067 g of iodine. What is the empirical formula? A. NiI B. Ni2I3 C. NiI3 D. NiI2 65. What is the percentage of hydrogen in C14H10? A. 5.67% B. 94.33% C. 12.01% D. 6.50% 61. What is the percentage of nitrogen in NH4NO3? A. 59.96% B. 5.05% C. 14.01% D. 35.00% 66. What is the percent of nitrogen in HNO2? A. 68.06% B. 29.80% C. 2.15% D. 14.01% 62. What is the formula mass for Fe2(SO4)3? A. 688.42 B. 886.24 C. 911.82 D. 399.88 67. What type of formula gives you the simplest whole-number ratio of atoms in a compound? A. ideal formula B. empirical formula C. molecular formula D. baby formula 63. What is the formula mass for C2H4? A. 13.02 B. 28.06 C. 14.02 D. 106.14 68. What is the percentage of hydrogen in NH4NO3? A. 59.96% B. 14.01% C. 35.00% D. 5.05% 69. What is the number of molecules in 1500 moles of N 2H4? A. 9.0 X 10 26 B. 6.02 X 10 23 C. 9.0 X 10 23 D. 6.02 X 10 26 29.A 30.A 31.B 32.B 33.D 34.D 35.D 36.C 37.A 38.C 39.C 40.C 41.C 42.D 43.A 44.B 45.D 46.A 47.D 48.C 49.C 50.A 51.C 52.B 53.B 54.C 55.C 56.C 57.A 58.C 59.D 60.D 61.D 62.D 63.B 64.C 65.A 66.B 67.B 68.D 69.A Answer Key 1. B 4. E 7. D 8. B 10. E 12. C 13. B 14. B 15. E 17. E 18. A 19. D 20. C 23. C 27. E 28. A Page 2









