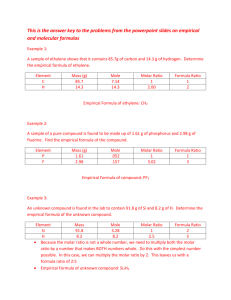
Percent Composition, Empirical Formula and Molecular Formula
advertisement
Percent Composition, Empirical Formula and Molecular Formula 10.4 summary Percent Composition 1 pizza • 2 slices pineapple • 6 slices pepperoni • 4 slices cheese What % of the pizza slices are • pineapple? • pepperoni? • just cheese? (2/12) x 100 = 17% pineapple, (6/12) x 100 = 50% pepperoni, (4/12) x 100 = 33% cheese Percent Composition of a Compound from experimental data • ex: a lab sample contains 24.02 g carbon and 64.00 g oxygen. element mass %composition = x100 compound mass • compound mass = 24.02 g + 64.00 g = 88.02 g • % C: (24.02 g / 88.02 g) x 100 = 27.29% C • % O: (64.00 g / 88.02 g) x 100 = 72.71 % O good because 27.29% + 72.71% = 100% Percent Composition of a Compound from a chemical formula • ex: find the percent composition of CO2. element mass in1molecompound %composition = x100 molar mass ofcompound • MM of C = 12.01 g/mole • MM of O = 16.01 g/mole • molar mass of compound = 12.01 + 2(16.00) = 44.01 g/mole • % C: (12.01 g / 44.01 g) x 100 = 27.29% • % O: (2X16.00 g / 44.01 g) x 100 = 72.71% good because 27.29% + 72.71% = 100% Empirical Formula • Empirical means determined experimentally. • If an unknown sample is analyzed and the amount of each element is determined, an “elementary” formula can be created. • smallest, whole-number ratio of moles of each element in the compound. • hydrogen peroxide = HO Calculating Empirical Formula 1. If given %, assume 100g sample. 2. Convert mass of each element to moles. 3. Divide each of these numbers by the smallest number to force one of them to = 1. 4. If necessary, multiply by the smallest number possible to make each a whole number. 5. These whole numbers are the subscripts in the empirical formula called mole ratio. empirical formula = XAYBZC Molecular Formula • Actual number of atoms of each element in a molecule or formula unit. • hydrogen peroxide = H2O2 • experimental molar mass needs to be provided. Calculating Molecular Formula 1. Find molar mass of empirical formula. 2. Divide experimentally determined molar mass (given), by molar mass of empirical formula. givenMM n= empiricalMM 1. Has to equal a whole number “n” 2. Multiply each subscript by n. molecular formula = XnxAYnxBZnxC