Table S1: Overview of projects in the CSER Consortium
advertisement
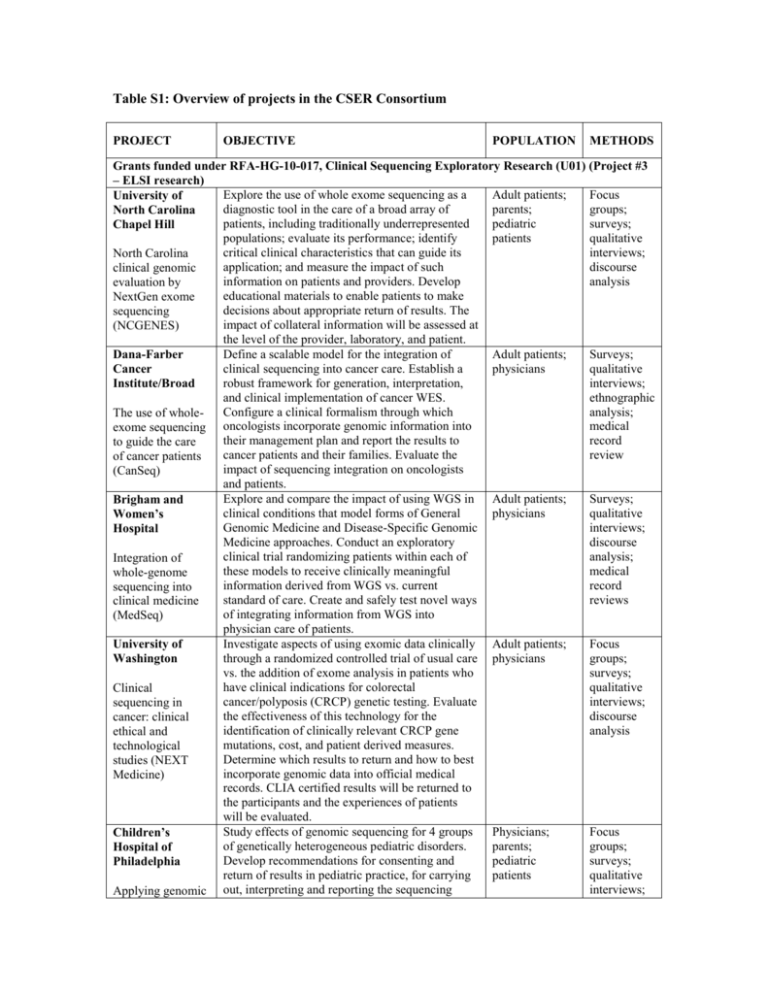
Table S1: Overview of projects in the CSER Consortium PROJECT OBJECTIVE POPULATION METHODS Grants funded under RFA-HG-10-017, Clinical Sequencing Exploratory Research (U01) (Project #3 – ELSI research) Explore the use of whole exome sequencing as a Adult patients; Focus University of diagnostic tool in the care of a broad array of parents; groups; North Carolina patients, including traditionally underrepresented pediatric surveys; Chapel Hill populations; evaluate its performance; identify patients qualitative critical clinical characteristics that can guide its interviews; North Carolina application; and measure the impact of such discourse clinical genomic information on patients and providers. Develop analysis evaluation by educational materials to enable patients to make NextGen exome decisions about appropriate return of results. The sequencing impact of collateral information will be assessed at (NCGENES) the level of the provider, laboratory, and patient. Define a scalable model for the integration of Adult patients; Surveys; Dana-Farber clinical sequencing into cancer care. Establish a physicians qualitative Cancer robust framework for generation, interpretation, interviews; Institute/Broad and clinical implementation of cancer WES. ethnographic Configure a clinical formalism through which analysis; The use of wholemedical exome sequencing oncologists incorporate genomic information into their management plan and report the results to record to guide the care cancer patients and their families. Evaluate the review of cancer patients impact of sequencing integration on oncologists (CanSeq) and patients. Explore and compare the impact of using WGS in Adult patients; Surveys; Brigham and clinical conditions that model forms of General physicians qualitative Women’s Genomic Medicine and Disease-Specific Genomic interviews; Hospital Medicine approaches. Conduct an exploratory discourse clinical trial randomizing patients within each of analysis; Integration of these models to receive clinically meaningful medical whole-genome information derived from WGS vs. current record sequencing into standard of care. Create and safely test novel ways reviews clinical medicine of integrating information from WGS into (MedSeq) physician care of patients. Investigate aspects of using exomic data clinically Adult patients; Focus University of through a randomized controlled trial of usual care physicians groups; Washington vs. the addition of exome analysis in patients who surveys; have clinical indications for colorectal qualitative Clinical cancer/polyposis (CRCP) genetic testing. Evaluate interviews; sequencing in the effectiveness of this technology for the discourse cancer: clinical identification of clinically relevant CRCP gene analysis ethical and mutations, cost, and patient derived measures. technological Determine which results to return and how to best studies (NEXT incorporate genomic data into official medical Medicine) records. CLIA certified results will be returned to the participants and the experiences of patients will be evaluated. Study effects of genomic sequencing for 4 groups Physicians; Focus Children’s of genetically heterogeneous pediatric disorders. parents; groups; Hospital of Develop recommendations for consenting and pediatric surveys; Philadelphia return of results in pediatric practice, for carrying patients qualitative interviews; Applying genomic out, interpreting and reporting the sequencing sequencing in pediatrics (PediSeq) data. Develop decision support tools for discourse integration into electronic health record. Assess analysis the outcomes of sequencing from the perspective of children, parents and primary care providers. Physicians; Surveys; Baylor College of Integrate CLIA-certified germline and tumor parents; qualitative Medicine exome sequencing information into the care of pediatric interviews; childhood cancer patients with high-risk solid patients discourse tumors and brain tumors. Assess the impact of Incorporation of analysis WES data reported by a novel web-based platform genomic with links to existing data for each variant sequencing into reported and presented through a graphical display pediatric cancer that will facilitate physician disclosure of complex care (BASIC3) data to parents. Perform quantitative analysis of physician communication in disclosure of genome-scale data, and assess parental understanding and preferences for receiving genome scale data. Grants funded under RFA-HG-11-003, Development of a Preliminary Evidence Base to Inform Decision-making about Returning Research Results to Participants in Genomics Studies (R01) Explore the extent to which research participants' Parents; Focus Boston preferences can reliably guide the return of genomic groups; Children’s individual genomic research results and how research qualitative Hospital participants' preferences regarding the return of participants interviews Returning research results can be incorporated into a governance results in children: structure for a genetic research registry or Parental biobank. preferences and expert oversight Genomic Surveys; Seattle Children’s Work with experts in clinical genetics, genomics, genetic counseling and biomedical informatics to research qualitative Hospital compare the use of traditional approaches for participants interviews Innovative returning research results (using face-to-face approaches to sessions with genetic counselors) to an innovative returning results in web-based tool. Based on the study findings, exome and develop a framework, guidelines and proposed genome policies for returning genome sequencing results sequencing studies to research participants. (My46) Investigate the preferences of participants who Genomic Surveys, Columbia have enrolled in genomic research studies with research qualitative University respect to getting back incidental genetic results, participants; interviews Impact of return of as well as the potential psychosocial and genomic incidental genetic behavioral consequences of receiving this researchers test results to information. Analyze a range of possible research incidental findings that could be reported and participants in the explore researchers' varying responsibilities to genomic era report these results based upon their clinical and psychosocial implications. Grants funded under RFA-HG-11-004, Ethical, Legal, and Social Implications of Returning Research Results to Genomic Research Participants (R21) Develop a menu of potential approaches for Genomic Surveys; Columbia dealing with the challenges of informed consent research qualitative University that must be addressed before widespread efforts participants; interviews to return genomic research results are put into genomic Challenges of place. Obtain the perspectives of genomic researchers informed consent in return of data from genomic research investigators and research participants, their suggestions for addressing them, and their thoughts about the various options. Analyze the normative issues involved and identify realistic options for future researchers. Identify criteria to guide decisions about returning Vanderbilt individual research results to children who University participate in genomic research. Examine U.S. law Returning research and international guidelines about decisionresults of pediatric making for and by minors. Analyze what weight should be given to various benefits potentially genomic research associated with returning pediatric research to participants results. Conduct philosophical research to analyze The Children’s critically the claim that it is morally obligatory, or Mercy Hospital at least morally permissible, for genomic The presumptive researchers who study samples in biorepositories case against to return individual research results participants. returning Identify and consider potential solutions for any individual results significant practical challenges that may be in biobanking encountered if a biorepository decides not to research return individual research results. Conduct research to develop the normative and Johns Hopkins legal framework that will be necessary to consider University if newborn screening programs are to be expanded Return of research and if efforts are to be made to offer to return results to parents for research conducted with results from dried blood spots. Evaluate existing state policies samples obtained on newborn screening and the development of for newborn biorepositories using dried blood samples, identify screening gaps in existing regulations, and develop recommendations for policymakers. Investigator-initiated grants Develop effective ethical strategies for offering University of genomic incidental findings to participants in a California San biobank for pancreatic cancer. The approach will Francisco/ Mayo Clinic/ University be informed by studying the preferences of biobank research participants and family members of Minnesota using mixed methods. Generate data on proband and family preferences; produce detailed analyses Disclosing genomic incidental of the legal, ethical and practical issues raised when incidental findings are identified among findings in a probands (both deceased and living); create cancer biobank: consensus recommendations, devise methods for An ELSI honoring preferences, and advance sound biobank experiment governance. Describe the attitudes and beliefs of patients and Cleveland Clinic Lerner College of genetic professionals regarding the types of Medicine of Case diagnostic possibilities that should be discussed with patients prior to large-scale clinical mutation Western Reserve testing, and to characterize the attitudes and University beliefs of patients and genetic professionals Presenting regarding the types of diagnostic results that diagnostic results should be returned following genomic testing. from large-scale Pediatric genomic research participants Legal analysis Genomic research participants Normative analysis Parents of newborn screening research participants Normative and legal analysis Genomic research participants; family members Surveys; qualitative interviews; return of results prototype experiment Adult patients; genetic professionals Surveys; qualitative interviews; working groups clinical mutation testing National Institutes of Health Intramural project Characterize intentions and choices to receive and ClinSeq® use different types of genomic sequencing National Human information, looking at key independent variables Genome such as attitudes toward sequencing, perceived Research benefits and risks. and uncertainty Institute, NIH Test interventions to return results and their affect Predictors of on desired outcomes including informed choice, preferences, decisional satisfaction, family communication and uptake and use of health care use. genomic sequencing information Adults (45-65 yrs) ranging in health status from healthy to affected with coronary artery disease Surveys; focus groups; qualitative interviews








