MSE 231H1F Characterization of Materials - Skule Courses
advertisement

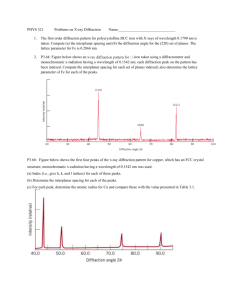
Last Name: First Name: Student #: MSE 231H1F Characterization of Materials 2004 / 2005 Quiz # 1 Time: 50 minutes Date: November 12, 2004 ______________________________________________________________________________ 2d sin d N Fhkl f n e 2 i ( hun kvn lwn ) a h2 k 2 l 2 [UVW ] (hkl ) hU kV lW 0 1 e i e3 i e5 i 1 e n i (1) n , where n is any in teger e2 i e4 i e6 i 1 e n i e n i , where n is any in teger b1 2 b 2 2 b3 2 a 2 a3 a1 a 2 a 3 a 3 a1 a1 a 2 a 3 a1 a 2 a1 a 2 a 3 bi a j 2 ij ij 0, for i j; ij 1, for i j c d c2 d3 c3d 2 , c3d1 c1d3 , c1d 2 c2 d1 c d c1d1 c2d2 c3d3 ______________________________________________________________________________ Answer all questions. The marks for each part are shown in the left margin. There is a total of 30 marks. You have 50 minutes to complete this quiz. Page 1 of 4 Last Name: First Name: Student #: The Ni-base superalloys have been designed for high temperature applications, e.g. turbine blades in jet engines. Ni3Al is an important strengthening phase in these materials. Its structure is shown below: a = 0.36 nm 10 1) Ni3Al has the L12 structure, with 1 Al atom located at 0 0 0 and 3 Ni atoms located at ½ ½ 0, ½ 0 ½, and 0 ½ ½. Derive the diffraction rules for this crystal using the structure factor. Fhkl f Al e2 i (0) f Ni (e2 i ( h / 2 k / 2) e2 i ( h / 2l / 2) e2 i ( k / 2l / 2) ) f Al f Ni (e i ( h k ) e i ( h l ) e i ( k l ) ) Case1: (mixed) Fhkl f Al f Ni (1 1 1) f Al f Ni Fhkl 2 f Al f Ni 2 Case2: (Unmixed) Fhkl f Al f Ni (1 1 1) f Al 3 f Ni Fhkl 2 f Al 3 f Ni 2 Page 2 of 4 Last Name: 2) Student #: Draw a schematic diagram of the powder X-ray diffraction pattern for the Ni3Al phase over the 2 range of 30 to 55. Label and give the diffraction angle for each peak in this range (use an X-ray wavelength of 0.154 nm and a lattice parameter of 0.36 nm). Use the following equations to find values of “d” and “2” a d h2 k 2 l 2 2d sin h2+k2+l2 1 2 3 4 5 Plane 100 110 111 200 210 12.35 17.6 21.7 25.3 28.57 d 0.360 0.255 0.208 0.180 0.161 2 24.70 35.21 43.48 50.65 57.14 1 0.8 Intensity 10 First Name: 0.6 0.4 0.2 0 30 35 40 45 50 55 2 theta (degree) Page 3 of 4 Last Name: First Name: Student #: 5 3) Draw a schematic diagram of the 9 central spots in the electron diffraction pattern of a single crystal of Ni3Al oriented with the [010] direction parallel to the incident electron beam. Label each diffraction spot. 5 4) TiO2 is polymorphic. One form of TiO2 is the mineral rutile, which is used extensively as a pigment because of its high refractive index (2.70). Rutile has the tP6 crystal structure. Derive the reciprocal lattice vectors for rutile using the following direct lattice primitive vectors: a1 axˆ; a 2 ayˆ ; a3 czˆ a2 a3 acxˆ a1 (a2 a3 ) a 2 c 2 acxˆ 2 xˆ b1 2 ac a a3 a1 acyˆ 2 acyˆ 2 yˆ a 2c a 2 a1 a2 a zˆ b2 b3 2 a 2 zˆ 2 zˆ a 2c c Page 4 of 4