Steps to Balancing Redox Reactions
advertisement

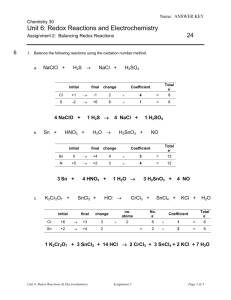
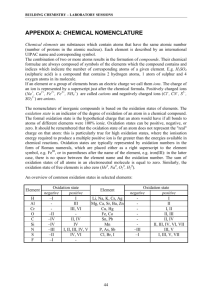
Steps to Balancing Redox Reactions 1. Write the unbalanced net ionic equation and look to see if the final solution is acidic or basic. 2. Identify and label the oxidation states of each atom in every species. Use the rules for prioritizing elements (see handout for oxidation states) if you are not familiar with this. 3. Identify the oxidation and the reduction half reactions. Make a note of how many e- are lost or gained by each species. 4. Write the separate half-cell reaction equations. To balance the atom number: a) Balance all atoms on each side (except O and H). b) Balance the number of oxygen atoms by adding water to the opposite side. c) Balance the number of hydrogen atoms by adding H+ to the opposite side. To balance the charge: d) Make the charges on each side of the equation the same by adding e-. (NOTE: this is a good place to check; the number of e- lost or gained in step 3 per atom should be the same as in the half-reactions.) e) If the number of electrons in each half-reaction is not the same, multiply by a factor so that both are the same. (remember, e- lost must = e- gained) 5. Combine half-reactions to make a full equation. Simplify by combining like terms. 6. Check to make sure that the atom number and the total charge on each side is the same. 7. If the solution is basic, add enough OH- to neutralize all the extra H+. These turn into waters on that side. Also add the same number of OH- to the other side of the equation. (Just like algebra; What you do to one side must be done to the other.) __________________________________________________________________ Some examples: 1. I-(aq) + Br2(l) IO3-(aq) + Br-(aq) (acidic) Hint: when balanced, coefficient in front of Br2 is a 3. 2. As2S3 + H2O2 AsO43- + SO42- (basic) Hint: when balanced, coefficient in front of H2O2 is 14 and OH- is 20.