Diffusion Experiment (Lee and Wilke Method)
advertisement

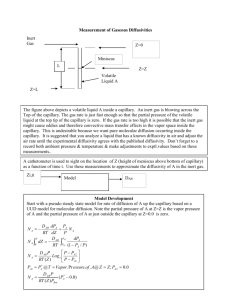
Diffusion Experiment (Lee and Wilke Method) Introduction This experiment is similar to that described by Lee and Wilke1. The theory for the special case of “species A” diffusing through stagnant “species B” is detailed in Geankoplis2. In our version, dry air is passed over 5 NMR tubes with a diameter of 7.0 mm. A cathetometer is used to visually track liquid levels over time. Constant air flow is from house air. The air is dried prior to passing over the tubes. Airflow is measured by a digital flow meter at the outlet. Linear regression as described by Lee and Wilke is used to account for uncertainty in actual diffusion path length. These uncertainties arise due to convective mixing in the mouth of the tubes and the meniscus at the gas-liquid interface. You will do this experiment as a class. Each group will alternated taking hourly readings over the course of the day. Figure 1- Diffusion apparatus. Air is dried by passing through a drying tube filled with DriRite absorbent. The tubing and manifold are made of copper to heat the air and provide a uniform temperature. Liquid levels measured with a cathetometer. 1 Lee, C.Y. and Wilke, C.R., Industrial and Chemical Engineering, p 2381, November 1954 (in-class handout). 2 Geankoplis, J.G., Transport Processes and Separation Principles, 4th ed. pp 417-420 Standard Operating Procedure 1. You will be given a bottle of either acetone or cyclohexane to use for this experiment. Check the MSDR for hazards of these substances. Use appropriate PPE (safety glasses and nitrile rubber gloves). 2. Use the syringe and fill tube provided to fill the NMR tubes to five different levels of your choosing. Fill from the bottom so as to keep surfaces above the liquid level from being wetted. 3. Insert tubes in the manifold such that they are all aligned perpendicular to the air flow and protrude into the manifold the same distance. 4. Place the manifold into the constant temperature bath (aquarium) and fill the bath to within one centimeter of the manifold (after the bath is heated you may top off the fluid so that it is within a few millimeters if the manifold). 5. Set the bath to the desired temperature and set the airflow to the desired rate and allow tocome to equilibrium. 6. Level the cathetometer using the thumbscrews on the feet of the device. Use duct tape to secure the cathetometer to the floor so that it is less likely to move if accidentally bumped. 7. Level the telescope using the thumbscrew on the scope. 8. Carefully take initial level measurements of all tubes by adjusting the height of the scope using the thumbscrew that controls the height (note that the readings are Repeat once per hour for 6 hours. Use extreme caution when adjusting the height during the day- if the apparatus is handled roughly or bumped, your measurements will not be accurate. Note that the final significant figure of your height is read from the Vernier scale. See http://en.wikipedia.org/wiki/Vernier for instructions on reading Vernier scales. 9. Repeat this experiment at a new temperature on day 2. Analysis 1. Calculate the apparent diffusivity for each tube using eqn. 6.2-262. Plot your data by modifying equation 6.26-26 in such a way that all of the time data is used and the diffusion coefficient may be obtained from the inverse slope. Is the data linear- are there any patterns? Why or why not? How long would you expect it to take for the system to come to steady state? What does scatter in the data represent? Use linear regression and statistical output (Excel or Polymath) to estimate the uncertainty in the value of diffusivity based solely on the the assumption of random scatter in the data. 2. Plot the apparent diffusivity values of each tube as in eqn 11 in Lee and Wilke in order to determine actual values of diffusivity. Use linear regression and statistical output (Excel or Polymath) to estimate the uncertainty in the value of diffusivity based solely on the the assumption of random scatter in the data. 3. Calculate the expected value of diffusivity using both the Chapman-Enskog equation (6.2-44) and the Fuller, Schettler and Giddings method (eqn 6.2-45, table 6.2-2 in Geankoplis). 4. Compare your experimental value of the diffusivity coefficient with the estimated values from number 2 above, as well as with the literature value for aircyclohexane or air-acetone. Are the results consistent? Is the temperature dependence between T1.5 and T1.75? 5. What systematic uncertainties are present as a result of the methods and materials you used to measure the diffusion coefficient? 6. What assumptions were made in the derivation of equation 6.2-26? How does the method outlined in the Lee and Wilke paper correct for some of the “nonidealities” of the system? (explain fully) 7. Show plots in items 1 and 2 in your “Results” section and discuss all items fully in the “Discussion”.