EXPERIMENT NO. 5-5
advertisement

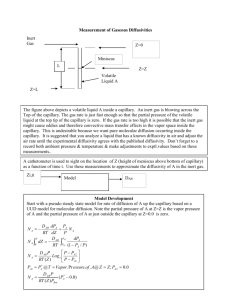
Chemical Engineering Laboratory Manual Diffusivity Measurement EXPERIMENT 461L_4 DIFFUSIVITY MEASUREMENT OBJECTIVES: 1. Understand Fick’s law as it relates to the unimolecular diffusion of a volatile organic compound in a stagnant column of air. 2. Measure and compare the evaporation rates for different volatile organic compounds in a stagnant column of air. 3. Determine the experimental diffusivity values (sometimes called diffusion coefficients) from your evaporation data. 4. Compare the experimental diffusivity value with values reported in the literature. 5. Compare the experimental diffusivity value with a value predicted by an empirical equation. 6. [Optional] Compare experimental model to Comsol simulation model. THEORY: Diffusion is the mass transfer of an individual component through a stagnant mixture due to a concentration gradient. The rate of diffusion is described by Fick’s law: J A DAB where JA DAB cA z dc A dz (1) = molar flux of A in the z direction, relative to the total flow [mol/cm2s] = diffusivity, [cm2/sec] = molar concentration of A [mol/cm3] = direction of concentration gradient [cm] In diffusion, mass transfer occurs via random movements at the molecular level. Note the similarity between Newton’s law of viscosity (momentum transport), Fourier’s law of conduction (heat transport), and Fick’s law of diffusion (mass transport). These laws describe transport of different entities via the same random molecular process. Note that the system must be stagnant for Fick’s law to apply; if bulk mixing or turbulence is present, the overall rate of mass transfer will be much greater than predicted due to convective transport. In this experiment, our concern focuses on gaseous diffusion. Diffusivities in gases can be predicted with considerable accuracy from kinetic theory, e.g., reference (2). The theoretical correlations have been modified in the light of experimental data to give the semi-empirical equation 1, shown below. It is equation (21.25), page 655, of reference (1). 0.01498T 1.81 1/M A 1M b 0.5 D AB pTcA TcB 0.1405 V 0.4 cA 0.4 VcB 2 461L_5 - 1 (2) Chemical Engineering Laboratory Manual Diffusivity Measurement In this equation, the units indicated must be used because of the empirical nature of the relationship. Here: MA, MB - p T TcA, TcB - molecular weights of components A & B, respectively pressure, atm. temperature, K critical temperatures of A & B respectively, K VcA, VcB - critical molar volumes of A & B respectively, cm3/ g mol Critical molar volumes are tabulated in handbooks and textbooks, or can be estimated from correlations in references (3,4,5,9, and 10). Other empirical equations are listed in references (1,2,5,6, and 10). EQUIPMENT: To measure diffusion of a volatile liquid or solid, an upright glass tube of known dimensions can be partly filled with the volatile substance. The evaporation tube should have a length to diameter ratio of at least 4.0 so that the diffusion path is at least 4 times the cylinder diameter. The material of construction of the cylinder should be inert to the liquid used. Evaporation tubes and various volatile organic compounds can be obtained from the lab teaching assistant. EXPERIMENTAL: Select two to three different volatile compounds for your diffusion experiments. The compounds chosen should have a normal boiling point or sublimation point above 50 C. It should not be soluble in water as this might result in the formation of a solution with water vapor from the air, changing its vapor pressure characteristics. It should have a molecular weight greater than that of air so as to prevent convection within the cylinder. Partially fill your glass tubes with volatile liquid/solid, and place your tubes upright in a well-ventilated, temperature controlled room. If a low temperature oven is available, try investigating diffusion as a function of temperature, however, use caution with flammable solvents, since many ovens are not explosion proof. Each substance should be run at least in duplicate. The mass transfer (evaporation) rate of the volatile compound from the tube can be measured by the change in weight, or by the change in height of the liquid. Monitor the amount of volatile substance remaining in each tube as a function of time over the course of several days. Note that the diffusion path length increases as evaporation proceeds. Write down the room/oven temperature each time you measure your tubes. 461L_5 - 2 Chemical Engineering Laboratory Manual Diffusivity Measurement SAFETY: Evaporation tubes should be placed in a fume hood or well ventilated room. Read the material safety data sheet (MSDS) for the substance chosen - it is located in the chemistry storeroom or on the WWW (World-Wide Web) (try http://research.nwfsc.noaa.gov/msels.html). REPORT: In writing up this experiment: 1. Plot your raw data as z or mass remaining as a function of time. Try to explain observed trends and differences between substances. 2. Calculate and compare the diffusion rate (evaporation rate) of the vapor through the diffusion path for the different substances. 3. Calculate the diffusivity of the vapor through the air from the experimental data. Assume unimolecular diffusion and refer to the appropriate equations in one of the references listed below. Use the slope of a best-fit line to determine a diffusivity value that best fits your data throughout the time/course. The uncertainty in the slope provides an estimate of the uncertainty in your diffusivity value. Discuss possible and probable sources of uncertainty. 4. Estimate the diffusivity of the vapor by means of the semi-empirical equation given above. 5. Compare your experimental diffusivity values with those obtained from equation 2 above. Also compare your results with published diffusivity values, corrected for temperature differences as needed. Discuss reasons for statistically significant differences, if any. QUESTIONS FOR THOUGHT/DISCUSSION: 1. Can you explain how the equation you used to calculate experimental diffusivity values was obtained from Fick’s law? 2. Can you explain differences in evaporation rates for different compounds knowing the physical properties for each compound? 3. Why is it important that the length to diameter ratio of the evaporation tube be greater than 4.0? 4. What is the difference between mass transfer by diffusion versus mass transfer by convection? Which process is faster? 5. What is the difference between unimolecular diffusion and equimolar counterdiffusion? REFERENCES: 1. Warren L. McCabe, Julian C. Smith and Peter Harriott, Unit Operations of Chemical Engineering, Fifth Edition, McGraw-Hill Book Co. (1993). 2. R.B. Bird, W.E. Stewart and E.N. Lightfoot, Transport Phenomena, pp 508-513, Wiley (1960). 461L_5 - 3 Chemical Engineering Laboratory Manual Diffusivity Measurement 3. R.H. Perry, C.H. Chilton and Sidney D. Kirkpatrick, Chemical Engineers' Handbook, Fourth Edition, pp. 14-19 to 14-24, McGraw-Hill Book Co. (1963). 4. Robert H. Perry and Cecil H. Chilton, Chemical Engineers' Handbook, Fifth Edition, pp. 3231 to 3-235, McGraw-Hill Book Co. (1973). 5. Robert H. Perry, Don W. Green and James O. Maloney, Chemical Engineers’ Handbook, Sixth Edition, pp. 3-256,257,285,286, McGraw-Hill Book Co., (1984). 6. C.J. Geankoplis, Transport Processes and Unit Operations, Third Edition, Prentice Hall PTR (1993). FOR ADDITIONAL INFORMATION: 7. N.H. Chen and D.F. Othmer, J. Chem. Eng. Data, 7, 37 (1962). 8. Robert E. Treybal, Mass Transfer Operations, Third Edition, pp. 21-35, McGraw-Hill Book Co. (1980). 9. Robert C. Reid, John M. Prausnitz and Bruce E. Poling, The Properties of Gases and Liquids, Fourth Edition, McGraw-Hill Book Co. (1987). 10. Anthony L. Hines and Robert N. Maddox, Mass Transfer - Fundamentals and Applications, pp. 1-26, Prentice Hall, Inc. (1985). 461L_5 - 4