WORKSHEET FOR CARBOXYLIC ACIDS AND ESTERS
advertisement

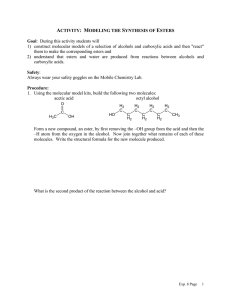
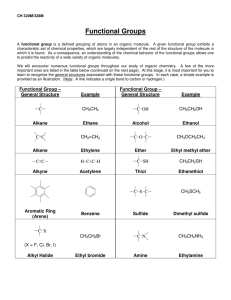
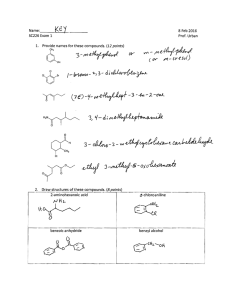
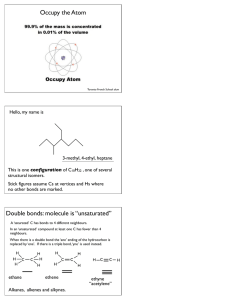
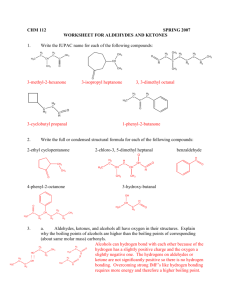
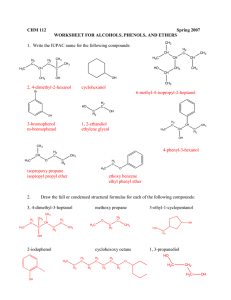
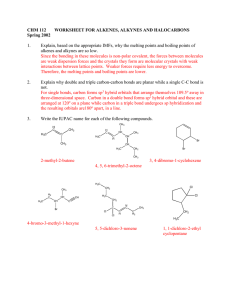
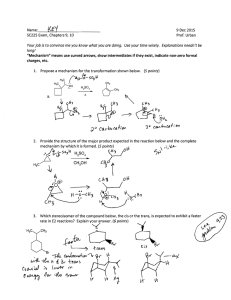
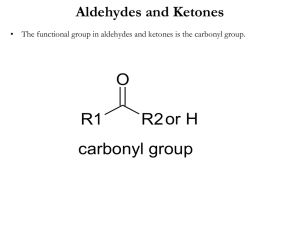
WORKSHEET FOR CARBOXYLIC ACIDS AND ESTERS 1. How is the geometry of the carbonyl group different from the geometry around a carbon in an alkane? Explain why. 2. Provide the IUPAC name for each of the following compounds. HO OH O Cl C C HO O _________________________________ _________________________________ OH H2 C O CH H2 C H3C CH H3C C C H2 OH C O OH _________________________________ O O _________________________________ O O C C H3C CH 3 _________________________________ H2 C O H2 C C H2 CH 3 _________________________________ 3. Using the Ka or pKa values, rank the acids in order from strongest (1) to weakest (2) acid. Acid Benzoic acid Trichloroacetic acid Acetic acid Pentanoic acid Hexanoic acid Rank Solubility Rank BP 4. Write the full or condensed structural formula for each of the following compounds. Butyric acid isopropyl hexanoate _________________________________ _________________________________ acetic acid cyclopentyl benzoate _________________________________ _________________________________ 2-hydroxy-3, 5-dimethyl heptanoic acid phenyl pentanoate _________________________________ _________________________________ 5a. Explain why acids are highly water soluble (even more so than corresponding alcohols). b. Explain what happens between carboxylic acid molecules that significantly increases their boiling points compared to comparable alcohols. 6. Complete the following reactions and supply the appropriate reactant(s) or product(s). + KOH OH C O HO CH 3 O CH 3 C + C H2 O CH ___________________ H3C C O C H2 O H2 C C O H2 C H2 C CH H3C H2O CH 3 LiAlH 4 OH C CH 3 O CH 3 CH 3 heat CH ______________________________ H2 C H3C H2 C C H2 H2 C O C H2 H3C C O CH3 NaOH CH O C C O O CH 3 CH 3