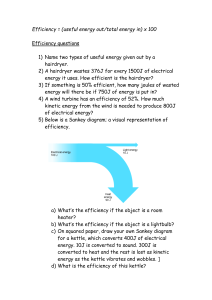
Heat and Temperature
Heat and temperature represent two different but related properties of matter. Heat
can be derived from the entire energy of a quantity of matter, which is the sum of the
kinetic and potential energies of each molecular or atomic constituent. Kinetic energy is
the energy associated with the motion of each particle of matter, and potential energy is
the energy stored in a particle as a result of its position or condition, as opposed to its
motion. Notice that there are no restrictions on the kinds of energy that can be heat. The
energy of an exothermic chemical reaction generates heat, as well as the energy E = mc2
derived from the energy content of matter in a nuclear reaction.
Temperature, on the other hand, is a measure of the average kinetic energy per
molecular or atomic constituent. Notice that two qualifiers are included in this statement:
temperature is related to the kinetic energy only; and temperature describes an average
property per constituent particle. Consider a large kettle of boiling water. If you measure
the temperature of the water, you will find that it is 100 °C. Suppose that you capture the
steam that is rising off the surface of the kettle and measure its temperature. You will find
that the temperature of the steam is also 100 °C. Even though the temperature of the
steam and the water are identical, the energy content per molecule of each is different.
The molecules of water in the steam are at a higher potential energy than the molecules of
water in the liquid water since it requires additional energy to overcome the molecular
attraction that binds water molecules together in liquid form. This is the reason that being
burned by steam at 100 °C is more damaging than being burned by water at the same
temperature. The kinetic energy of the molecules are identical, but the potential energy of
the steam is higher. Temperature is related to the kinetic energy only.
Next, consider a large kettle of water and a small teapot of water. Suppose that the
small teapot has one-fourth the volume of the large kettle. Starting from the same
temperature, it takes more energy to boil the large kettle of water than it takes to boil the
small teapot of water. For every molecule of water in the small teapot that has an increase
in kinetic energy, there are four molecules in the large kettle that require the same
average increase in kinetic energy. It requires four times as much energy, and therefore
four times as much heat, to excite the molecules in the large kettle to the same
temperature as that of the small teapot. Thus, heat is a measure of the total energy, while
temperature is an average property per molecule or atom.
The American Heritage® Book of English Usage. Copyright © 1996 by Houghton Mifflin Company. All rights
reserved.