KMT whiteboard answers
advertisement

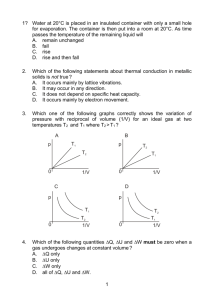
Just KMT Things Whiteboard Answers 1. What are the four ideas of the kinetic molecular theory? a. Gases consist of particles that are in random, rapid motion b. Kinetic energy of the gas is directly proportional to temperature c. Gases collide with each other and their container with no loss of energy (elastic) d. Gas particles occupy no volume relative to the overall volume of the container itself 2. Which two of the ideas in #1 are not technically true? c&d 3. Two equal balloons are made; one with Helium and one with Neon. How much faster does the Helium balloon deflate compared to the Neon? 2.25 times as much 4. SO2 diffuses 1.5 times as fast as an unknown gas. What is the molar weight of the unknown gas? 144 g/mole 5. You have four gas samples: a. 1 L H2 at STP b. 1 L of Ar at STP c. 1 L of H2 at 27 oC and 760 mm Hg d. 1 L of He at 0 oC and 900 mm Hg i) Which sample has the most atoms or molecules? Sample D ii) Which sample has the least atoms or molecules? Sample C iii) Which sample has the largest mass? Sample B iv) Which sample has the largest KE? Sample C 6. Five identical balloons are each filled to the same volume at the same temperature and pressure but are filled with CO2, O2, He, N2, and CH4. a. Which has the greatest mass? CO2 b. Compare the KEavg of the molecules in each of the balloons. Explain. They are all the same as they are at the same T c. All of the balloons decrease in size over time. Which balloon will be the smallest? Why? He will effuse through the balloon fastest as it is the smallest 7. CO and CO2 are put in separate containers of the same size and the same temperature. The pressure of CO gas is 2 atm while the pressure of the CO2 gas is 1 atm. a. Compare the KEavg of the 2 samples. Explain. KE is the same as both are at same T b. Which container contains a greater number of molecules. Justify your answer. CO is at same conditions but has 2 atm while CO2 has only 1 atm. The extra pressure must be coming from extra molecules 8. Why does the pressure of a gas go up when the temperature does? Pressure is defined as force over area. When temperature increases, the gas molecules have more kinetic energy, meaning they hit the walls of the container harder and more often, thus increasing force, while area remains constant. Since force is in the numerator, an increase in force will cause the pressure to increase. 9. What is true about gases at the same temperature? They have the same kinetic energy. Kinetic energy is. If a very massive molecule and a very light molecule have the same kinetic energy, the massive molecule will have a very low velocity while the light molecule will have a high velocity. 10. Why is a decrease in volume accompanied by an increase in pressure? This happens because there is less room for molecules to bounce against the walls of the container that they’re in so these molecules have to bounce against the walls more often. If pressure is force/area, then area is getting smaller while force increases, so pressure has to increase.