doc
advertisement
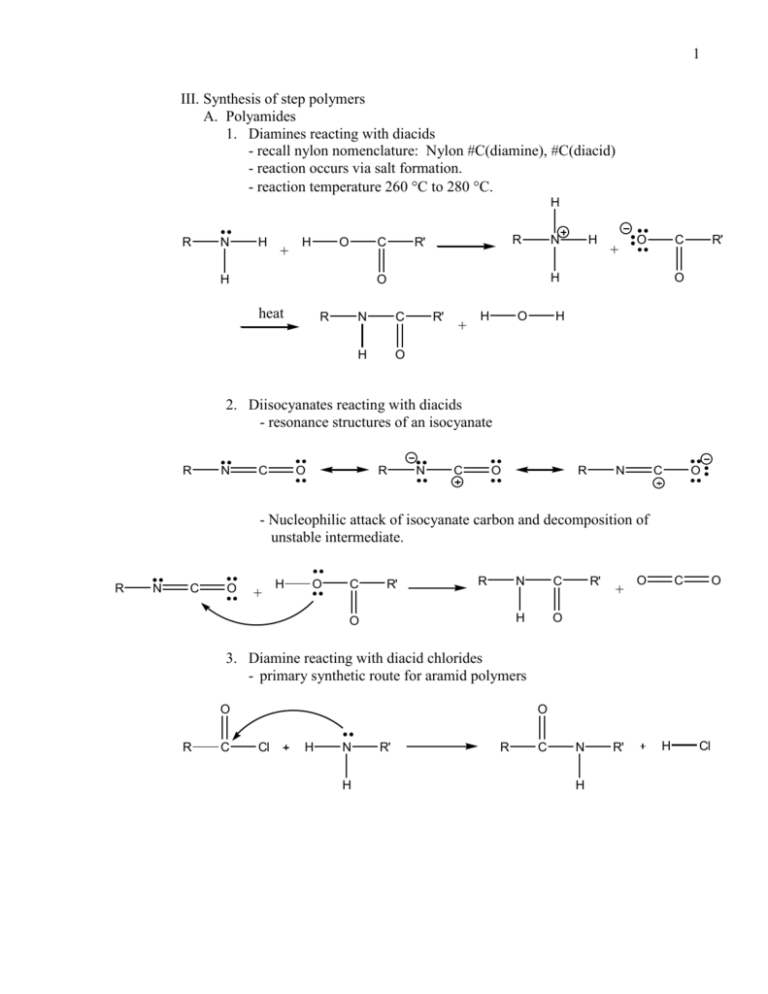
1 III. Synthesis of step polymers A. Polyamides 1. Diamines reacting with diacids - recall nylon nomenclature: Nylon #C(diamine), #C(diacid) - reaction occurs via salt formation. - reaction temperature 260 C to 280 C. H R N H + H O C H R R' N R + O C H O heat H N C H O R' + H O R' O H 2. Diisocyanates reacting with diacids - resonance structures of an isocyanate R N C O R N C O R N C O - Nucleophilic attack of isocyanate carbon and decomposition of unstable intermediate. R N C O + H O C R' R O N C H O R' + O C O 3. Diamine reacting with diacid chlorides - primary synthetic route for aramid polymers O R C O Cl H N H R' R C N H R' H Cl 2 4. Dicyanides reacting with diols R C N H + O H R' R C N + R' OH H2O R C N R' C N R' N R' O OH R R C N R R' C B. Polyesters 1. Diols reacting with diacids a. also known as an alkyd resin. H b. reversibility of esterfication is a problem. c. no salt intermediate formation d. high temperature synthesis yields limited enhancement of polymer synthesis. R O H + H O C R R' O C R' + H O H H2 C O O 2. Diols reacting with diacid chlorides - not nearly as reversible as diol/diacid reaction Cl O + O H H2 C O H2 C O H Cl R O O O O H2 C H O (Synthesis of PET) 3. Diesters reacting with diols - create diester of diacid through solvolysis R O H + H3C O C O R' R O C O R' + CH3 R' 3 4. Dianhydride with diol a. reaction with triol (e.g. glycerol) yields a glyptal resin (common for polyester paints) b. glycerol is an example of a crosslinking agent. O O O O + H O R' H O C O R C O R C. Polycarbonates 1. Diol reacting with phosgene (diacid chloride of carbonic acid) O H2 C HO + OH Cl C Cl Bisphenol – A O R H2 C O O R' 2. Diol reacting with bischloroformate O H O R' O O + H Cl C O R O C Cl O O O C O R O C O R' 3. Diol reacting with dichloride and carbon dioxide H O R O H + Cl R' Cl + O C O O O C O O R O C O R' R' 4 D. Polyurethanes (amide of carbonate) 1. Diol reacting with diisocyanate H O R O + O H C N R' N C O O O O H N C H N R' C O R a. Crosslinking is likely due to the reaction of isocyanate with urethane to form an allophanate. O O O H N C R' N C C O O R NH R' 2. Diamine reacting with bischloroformate O H2N R' NH2 O + Cl C O R O C Cl O H N C O O R O C H N R' 5 E. Polyureas (diamide of carbonate) 1. Diisocyanate reacting with diamine H2N R' + NH2 O C N R' N C O O H N O H N C H N R H N C R' 2. Diamine reacting with phosgene O O H2N R NH2 + Cl C H N Cl H N C R 3. Diamine reacting with carbonate O H2N R NH 2 + R' O C O R' O H N C H N + R' R OH F. Polysulfides 1. Dichlorides reacting with sodium polysulfide Cl R Cl R + Na2Sx + NaCl Sx 2. Dihalides reacting with dithiols (base catalysed) X R X + H S R' S H R S R' S 6 G. Polyethers 1. Dehydration of diols H O R O H H O O R R O O H R 2. Diol reacting with methylene chloride and base H O R O H + CH2Cl 2 O R H2 C O 3. Diol reacting with dibromide (with a phase transfer catalyst) H O R O H + Br R' Br O R O R' H. Polysulfones 1. Bischloroalkyl(aryl)sulfone reacting with diol and base O Na O R O Na + Cl R' S R' Cl O O O R O R' S R' O a. This is actually a polyether and a polysulfone. 2. Sulfonyl chloride in a Friedel-Crafts reaction AlCl 3or R SO2Cl O O FeCl 3 + R SO2Cl S O R S O R 7 I. Polyimides 1. Diamine reacting with dianhydride O O Pyromellitic anhydride H2N R NH 2 + O O O O O O N N R O O 2. Diisocyanate reacting with dianhydride O O O C N R N C + O O O O O O O N N O O R 8 J. Phenol-Formaldehyde resins 1. Resoles (base catalyzed) a. Resonance forms of the phenolate ion O O O O b. Addition of the formaldehyde occurs at the ortho and para positions to form methylolphenol (dimethylolphenol, trimethylolphenol). O O H C O H H 2 C O H O HOH 2C CH 2OH CH 2OH O CH 2OH 9 c. Methylolphenol combines with phenolate via an SN2 mechanism. Phenolate is the nucleophile and the methylol carbon is the electrophile. O OH O O C O CH 2OH H HH CH 2OH C H2 O O CH 2OH C H2 d. Also methylolphenol combines with phenolate via a Michael addition. O O CH 2 CH 2OH O O O O H CH 2 C H2 O O C H2 10 e. Workable product (with no crosslinking) is called A-stage resole. A-stage is soluble in many solvents. f. Curing at high temperature results in methylene bridging via protonation. g. Curing at low temperature and relativity neutral conditions may result in ether linkages. h. After curing, polymer is known C-stage resole. C-type resole is insoluble 2. Novolacs (acid catalyzed) a. Acid catalysis causes carbocation formation in formaldehyde. H H C C OH OH H H b. Para/para linkage is preferred at pH 3. c. Ortho/ortho linkage preferred at pH 4 – 5. d. Under strongly acidic conditions, ortho/ortho linkage is directed with using Zn2+ and Cd2+ catalyst. OH OH H OH CH 2OH C OH H CH 2OH 11 e. Crosslinking does not occur in novolacs without the addition of a crosslinker like paraformaldehyde or hexamethylenetetramine. i.) hydroxymethylol groups cannot crosslink. HO O H2 C O H2 C H2 C O N H2C O H2 C O H2 C H CH 2 CH 2 N CH 2 N C H2 N CH 2 f. The molar ratio of formaldehyde to phenol must be less than one for novolac formation. (Ratio also heavily affects molecular weight.) g. A molar ratio of formaldehyde to phenol greater than one promotes the synthesis of resole resins (with crosslinking). 12 K. Melamine-Formaldehyde resins 1. Melamine is formed from cyclic trimerization of cyanamine. H2N 3 N C N NH 2 NH 2 N N NH 2 2. Reaction with formaldehyde forms methylolmelamines (up to hexa). CH 2OH HOH 2C N CH 2OH N N N CH 2OH N N HOH 2C CH 2OH 3. Condensation (with acid catalysis) between methylol group and amine group yields highly crosslinked polymer. CH 2OH HOH 2C N CH 2OH N N N H CH 2OH HOH 2C CH 2OH N N N N N CH 2OH HOH 2C CH 2OH HOH 2C CH 2OH N N HOH 2C N N N N CH 2OH CH 2OH CH 2OH N N N CH 2 N N N HOH 2C CH 2OH N N N CH 2OH HOH 2C CH 2OH CH 2OH 13 L. Urea-Formaldehyde resins 1. Under basic conditions, formaldehyde adds to urea to form mono- and dimethylol derivatives. O H2N C H NH 2 C O O H N HOH 2C H N C CH 2OH H 2. Under acidic conditions the methylol derivatives are in equilibrium with imine. O H2N H N C H H C O O H H2N C N CH 2 H 3. The imine can trimerize to cyclic triazine NH 2 C O 3 H2N C O N N H2C CH 2 CH 2 O O N C H2N N C H2 C NH 2 4. Further condensation between methylol groups and amide groups yields a highly crosslinked polymer.