Homework 03 for Lecture 04
advertisement

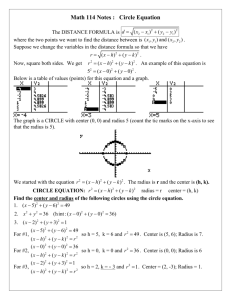
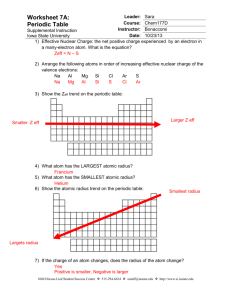
Homework #3 Lecture 4 Name _____________________ Use this to review Lecture 4. Turn in the answer sheet next class. 1. A cation is touched by 6 anions. What is the coordination number of the cation? a. 6 b. 8 2. In the above situation, the anions are arranged in a(n) a. octahedron b. tetrahedron. 3. Consider the mineral halite, NaCl , aka “salt”. Na+ is found to have a radius of 0.95 angstroms, and Clz has a radius of 1.81 angstroms. Calculate the radius ratio. a. 0.525 b. 1.91 4. For Halite, what coordination number do you expect the Na+ to have, i.e., how many Chloride Cl- ions are touching it? (Page 72) a. 6 b. 8 5. What coordination number is a body-centered cube BCC? a. 12 b. 8 6. In pure zinc Sphalerite, ZnS, Zinc ion Zn++ has a radius of 0.60 angstroms, and Sulfide ion S—has a radius of 1.84 Angstroms. What packing geometry do you expect? (Page 72 and 85) a. tetragonal (C.N. = 4) b. octagonal (C.N. = 6) 7. Calculate the electrostatic valency for Sphalerite (Pauling’s Rule 2) a. 0.5 b. 1.0 Geology 3261 Homework 3 ANSWER SHEET Name: __________________________ Homework assignments may be discussed with classmates Circle your answer: 1. a or b 2. a or b 3. a or b 4. a or b 5. a or b 6. a or b 7. a or b