Worksheet 7a answers - Iowa State University
advertisement
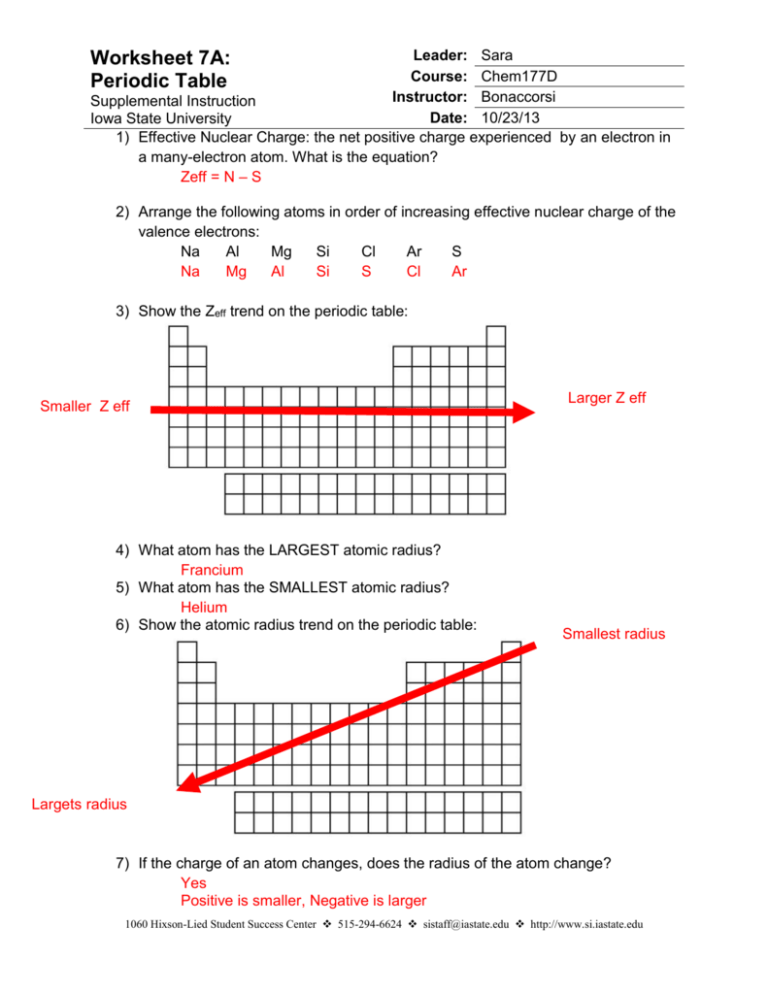
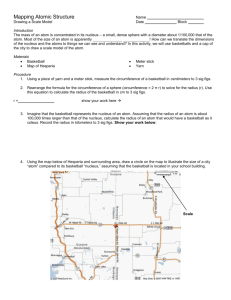
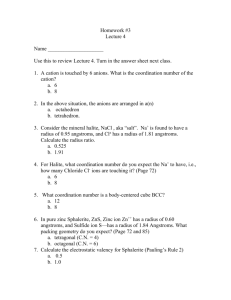
Leader: Sara Course: Chem177D Instructor: Bonaccorsi Supplemental Instruction Date: 10/23/13 Iowa State University 1) Effective Nuclear Charge: the net positive charge experienced by an electron in a many-electron atom. What is the equation? Zeff = N – S Worksheet 7A: Periodic Table 2) Arrange the following atoms in order of increasing effective nuclear charge of the valence electrons: Na Al Mg Si Cl Ar S Na Mg Al Si S Cl Ar 3) Show the Zeff trend on the periodic table: Smaller Z eff 4) What atom has the LARGEST atomic radius? Francium 5) What atom has the SMALLEST atomic radius? Helium 6) Show the atomic radius trend on the periodic table: Larger Z eff Smallest radius Largets radius 7) If the charge of an atom changes, does the radius of the atom change? Yes Positive is smaller, Negative is larger 1060 Hixson-Lied Student Success Center 515-294-6624 sistaff@iastate.edu http://www.si.iastate.edu 8) Rank the following in order of size smallest to largest: a. Te S O Po Se O S Se Te Po b. Ra O Ca P P Ga Ga Ca Fr Ra O Fr 9) Which is larger? a. Mg or Mg2+ b. Al or Al3+ c. I or I- d. K or K+ e. Se or Se2- f. Cl- or Br- g. F- or Ne h. N3- or P3- 10) What is isoelectronic? Elements/ ions with the same number of electrons 11) Put the following in order of increasing radius: BrSe2- Sr2+ Kr Rb2Se Br Kr Rb Sr2+