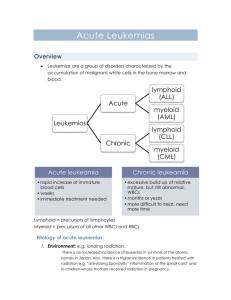
Neoplastic proliferations of white cells
advertisement

Doc. MUDr. L. Boudová, Ph. D. Myelodysplatic syndrome Chronic myeloproliferative disease Acute myeloid leukemia Abbreviations: G-granulopoiesis, E-erythropoiesis, BM – bone marrow, PB – peripheral blood; MDS – myelodysplastic syndrome; MPD – myeloproliferative disease; AL: acute leukaemia; AML – acute myeloid leukaemia; ALL – acute lymphoblastic leukaemia; CML – chronic myeloid leukaemia; PV – polycythemia vera; OMF - osteomyelofibrosis, ET – essentail thrombycythemia NEOPLASTIC PROLIFERATIONS OF WHITE CELLS - myeloid, lymphoid, histiocytic Myeloid neoplasms from the hematopoietic stem cells that give rise to cells of myeloid lineage -Thrombocytic - Granulocytic - Erythroid Acute myelogenous (myeloid) leukaemias (AML)– immature progenitor cells accumulate in the bone marrow Myelodysplastic syndromes (MDS) – ineffective hematopoiesis and cytopenia in the peripheral blood Chronic myeloproliferative disorders (CMPD)– increased production of one or more terminal differentiated myeloid elements – elevation of PB counts Myelodysplastic / myeloproliferative disorders – mixed features of MDS and CMPD Myelodysplastic synrome clonal disorders of stem cells defects of maturation in the BM - ineffective hematopoiesis (progressive failure of BM function) – cells in the PB : decreased numbers – pancytopenia + defective in function, pathological shapes Basic morphology: 1 Doc. MUDr. L. Boudová, Ph. D. BM: hypercellular, increased numbers of cells in the series – but: dysplastic: pathological forms, deranged architecture, the count of blasts may be increased – but less than 20%; which is the threshold between AML and MDS – NEW IN THE WHO CLASSIFICATION 2001 Examples: E: ringed sideroblasts, megaloblasts G: lack of nuclear segmentation M: lack of nuclear lobulation according to morphology: 5 subcategories possible progression to AML - various percentage according to subgroups (10 – 40%) primary. – de novo -old people – over 60 years secondary – therapy related – toxic exposure - worse prognosis – when it appears – progresses more frequently and more quickly to AL Therapy: symptomatic; some: bomne marrow transplantation Subcategories Refractory anemia – refractory = difficult to treat – unilineage dysplasia – affecting the E series RA with ringed sideroblasts – the nucleus encircled by siderotic granules (iron stained smears) RA with multilineage dysplasia RA with excess blasts –more than 5%, less than 20% MDS unclassifiable – lacks the more specefic abnormalities of other categories MDS assoc. with isolated del (5q) chromosome abnormality – long survival Chronic myeloproliferative disorders 2 Doc. MUDr. L. Boudová, Ph. D. CML, PV, OMF, ET – clonal disorders, affect typically adults Common principles: 1. stem cell genetic abnormalities, neoplastic proliferations affect one or more (all) BM myeloid series (red, white, megakaryocytes) – the disorder of an individual series is more pronounced ineach disease category – CML 2. neoplastic cells in the circulation – relatively normal maturation (difference when compared to MDS – ineffective maturation with cytopenia), 3.: splenomegaly, hepatomegaly : due to. sequestration of excess blood cells, extramedullary hematopoiesis, leukaemic infiltration - cells home to hematopoietic organs typicallyphases of the disease - development in time: onset is often insidious 1. proliferative phase, then: progression - -spent phase osteomyelofibrosis, or to blast phase 2. or to blast phase - all can progress to AL (CML does it invariably) – blast phase does not have to occur in all MPD (very rare in PV) Chronic myelogenous leukaemia (CML) distinctive translocation – reciprocal – t(9; 22) –long arms – Philadelphia chromosome; bcrabl gene fusion pluripotent stem cell defect abnormal fusion protein - increased tyrosine kinase activity The most striking feature: proliferation of G – increased cellularity – up to 100% of BM! maturation retained – all stages of G (no hiatus leukaemicus), hematopoiesis also extramedullary – splenomegaly (hepatomegaly) 3 Doc. MUDr. L. Boudová, Ph. D. PB: leukocytosis – exceeds even 100 000/ mm3 Phases: 1. chronic – aver. 3 ys 2.accelerated – gradual failure of response to treatment, increasing anemia and thrombocytopenia, basophilia 3. blast crisis –after accelerated phase or without the acceler. phase AL – 70% myeloid, the rest: lymphoblasts (mostly B) Ther. allog BMT, interferon A PV (polycytemia vera) increased proliferation of all three series – most striking: red cell mass increase. – in BM and PB hypercellular BM PB: HTC 60%.., Hb 180 and more – symptoms: increased RC mass - hypervolemia, blood stasis in vessels – mostly in venous circulation frequently cyanosis – stagnation and deoxygenation of blood hypertension, thrombotic episodes, bleeding – abnormal blood flow, abnormalities of PLT granulopoiesis may be elevated plt elevated + functional abnormalities Methods of treatment – phlebotomies - being abandoned. SPENT PHASE – myelofibrosis with myeloid metaplasia (in 20% after 10 ys) ET (essential thrombocytemia) the least common CMPD PLT exceed 600 000 /mm3 BM: increased cellularity, mega: abnormal, often large PLT: often large 4 Doc. MUDr. L. Boudová, Ph. D. Symptoms: thrombosis and hemorrhage – abnormalities of quantity and quality of PLT rel. indolent Chronic idiopathic myelofibrosis abnormal neoplastic megakaryocytes – release fibrogenic factors – PDGF and TGF stimulate fibroblasts to proliferation early: BM hypercellular with minimal fibrosis progression : hypocellular , fibrotic; osteosclerosis obliteration of BM space: extramedullary hematopoiesis - spleen; later: liver PB: leukoerythroblastic erytrhoid and granulocytic precursors in the PB in up to 20% - progression to AML Acute leukaemia Remember: all acute leukemias share some clinical similarities (namely acute course). They are discussed separately in these notes according to their progenitor cells). Acute leukemias: myeloid (+further subdivision according to the genetic features, morphology, immunophenotype); affect typically adults lymphoblastic (subdivision: B or T, +further subdivision according to the genetic features, morphology, immunophenotype) affect typically the young (children, adolescents) acute myeloid leukaemia (AML) the absolutely necessary minimum is to know why AML is dangerous, what cells it may be composed of, to know some of the categories) Immature precursors of the myeloid series (mostly G; less commonly also erythroid series or megakaryocytes) Typically: adults (only 20% of AL of children – children. typically ALL)) neoplastic cells may contain genetic alterations – acquired or inherited BM: neoplastic cells – inhibition of terminal differentiation - fill the BM – suppress normal hematopoiesis – physical replacement 5 Doc. MUDr. L. Boudová, Ph. D. - other mechanisms Problems – symptoms result from: failure of normal hematopoiesis – anemia, neutropenia, thrombocytopenia infiltration of organs by neoplastic cells BM: more than 20% of blasts Myeloblasts: nucleus: chromatin is delicate, more cytoplasm than lymphoblasts Azurophilic granules- peroxidase positive Red staining Auer rods- abnormally large azurophilicgranules Monoblasts: no Auer rods, folded nuclei Aleukaemic leukaemia – paradoxical term – BM filled by neoplastic cells but no neoplastic cells in the peripheral blood ("white blood without white blood") rare Classification – WHO 2001 – only some categories mentioned an effort to categorize them according to the genetic features, not only according to their appearance –such classification should be the most precise – the idea: to target the therapy at specific sites of the cell cycle (but not all of these facts are known currently) Categories: AML with recurrent gen. abnormalities – -balanced translocations, often complete remission, favourable prognosis (translocation – fusion gene-chimeric protein); e. g. t(15, 17) – AML M3 – promyelocytic – treatment with transretinoic acid; t(8, 21) or inversion of chromosome 16 AML therapy related AML with multilineage dysplasia AML – NOS- minim.differentiated Without maturation With maturation FAB classification = French – American – British (older than WHO) now used to categorise only cases which do not fall into the previous categories M0 – without maturation – myeloblastic 6 Doc. MUDr. L. Boudová, Ph. D. M1 – without maturation M2 – with maturation M3 – promyelocytic – nowadays categorised rather according to the genetic features – t(15; 17) M4 – myelomonocytic M5 – a – monoblastic, b- monocytic M6 – erythroid M7 – megakaryoblastic Myeloid sarcoma (granulocytic s., chloroma) solid tumour mass of AML cells - composed of myeloblasts or immature myeloid cells in an extramedullary site or in bone precedes or occurs concurrently with acute or chronic myeloid leukaemia (or MDS; CMPD) chloroma – because some are green prognosis: - when a part of AML: like AML when completely isolated - may be cured by radiotherapy 7