(IIV) Protocol Children Age 6 Months Through 8 Years
advertisement

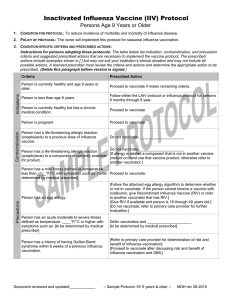
Inactivated Influenza Vaccine (IIV) Protocol Children Age 6 Months Through 8 Years 1. CONDITION FOR PROTOCOL: To reduce incidence of morbidity and mortality of influenza disease in children age 6 months through 8 years. 2. POLICY OF PROTOCOL: The nurse will implement this protocol for influenza vaccination. Contraindications Indications 3. CONDITION-SPECIFIC CRITERIA AND PRESCRIBED ACTIONS: Instructions for persons adopting these protocols: The table below list indication, contraindication, and precaution criteria and suggested prescribed actions that are necessary to implement the vaccine protocol. The prescribed actions include examples shown in [ ] but may not suit your institution’s clinical situation and may not include all possible actions. A licensed prescriber must review the criteria and actions and determine the appropriate action to be prescribed. (Delete this paragraph before version is signed.) Criteria Prescribed Action Child is currently healthy and age 6 months to 9 years Proceed to vaccinate if meets remaining criteria. Child is less than 6 months of age. Do not vaccinate. [Instruct parent to return when child turns age 6 months] [Encourage parents to get vaccinated.] Child is 9 years or older and healthy. Follow either the LAIV protocol or the influenza protocol for persons 9 years and older. Child is currently healthy but has a chronic medical condition. Proceed to vaccinate if meets remaining criteria. Child had a life-threatening allergic reaction (anaphylaxis) to a previous dose of influenza vaccine. Do not vaccinate; _____________________ Child has a life-threatening allergic reaction (anaphylaxis) to a component of currently available IIV product. Do not vaccinate; _____________________ [If allergy is related a component that is not in another vaccine product on hand use that vaccine product, otherwise refer to another vaccinator.] Precautions Child has a mild illness defined as temperature less than ____°F/°C with symptoms such as: Proceed to vaccinate. [to be determined by medical prescriber] Child has an acute moderate to severe illness defined as temperature ____°F/°C or higher with symptoms such as: [to be determined by medical prescriber] Defer vaccination and [to be determined by medical prescriber] Child has an egg allergy or allergy testing is suggestive of egg allergy even though the person has never been exposed to eggs. [Follow the algorithm for evaluation of an egg allergy to determine whether or not to proceed with vaccination.] [Do not vaccinate; refer to primary care provider for evaluation of whether or not to vaccinate.] Child has a history of having Guillan-Barré syndrome within 6 weeks of a previous influenza vaccination. Defer vaccination and ____________________________ [Refer to primary care provider for determination of risk and benefit of influenza vaccination] [Proceed to vaccinate after discussing risk and benefit of influenza vaccination and GBS.] Document reviewed and updated:____________ – Sample protocol: IIV 6 mo thru 8 yrs – MDH rev 8-2015 Sample - Trivalent Inactivated Influenza Vaccine (TIV) Protocol - Children Age 6 months through 8 Years 4. PRESCRIPTION: Give any of the following products that meet the age indication and administer according to dose and route described. Product* Dose Route Age Indication Fluzone, trivalent (IIV3) or quadrivalent (IIV4) 0.25 mL IM 6 months through 35 months Fluzone, trivalent (IIV3) or quadrivalent (IIV4) 0.5 mL IM 36 months or older Fluarix, quadrivalent (IIV4) 0.5 mL IM 3 years (36 months) or older Fluvirin, trivalent (IIV3) 0.5 mL IM 4 years or older FluLaval, quadrivalent (IIV4) 0.5 mL IM 3 years (36 months) or older *Use of product names are intended to help users delineate specific product indications and are not intended to be an endorsement of any particular product. Follow the attached algorithm in order to determine which children age 6 months through 8 years need a second dose of influenza vaccine. Give the 2nd dose at least 4 weeks after the first dose. 5. MEDICAL EMERGENCY OR ANAPHYLAXIS: [Depending on clinic staffing, include one of the two options below.] In the event of a medical emergency related to the administration of a vaccine. RN will apply protocols as described in ____________________________________________________________________________________________. In the event of an onset of symptoms of anaphylaxis including: o rash o itchiness of throat o difficulty breathing o bodily collapse o swollen tongue or throat LPN or unlicensed assistive personnel (MA) will immediately contact the RN in order to implement the ____________________________________________________________________________________________. 6. QUESTIONS OR CONCERNS: In the event of questions or concerns, call ____________________________at _____________________________. This protocol shall remain in effect for all patients of ______________________________until rescinded or until _____________________________________. Name of prescriber: _______________________________________________________________________________ Signature: ________________________________________________________________________________________ Date: ___________________________ Document reviewed and updated:____________ – Sample protocol: IIV 6 mo thru 8 yrs – MDH rev 8-2015 Algorithm for Evaluation of an Egg Allergy Preceding Influenza Vaccination, 2015 –16 Can the person eat lightly cooked egg (e.g., scrambled egg) without reactions? 1, 2 Yes Give vaccine per usual protocol. No After eating eggs or eggcontaining foods, does the person experience ONLY hives? Yes Give IIV if indicated and observe for reaction for at least 30 minutes after each dose.3 OR Give Recombinant Influenza Vaccine (RIV) if patient is 18 or older No Does the person experience other symptoms such as Cardiovascular changes (e.g., hypotension)? Respiratory distress (e.g., wheezing)? Gastrointestinal (e.g., nausea/vomiting)? Reaction requiring epinephrine? Give RIV if patient is 18 or older Yes OR IIV may be administered by a health care provider with advanced expertise in the management of allergic reactions (e.g., an allergist) Reaction requiring emergency medical attention? 1 Persons with egg allergy might tolerate egg in baked products (e.g., bread or cake). However, tolerance to egg-containing foods does not exclude the possibility of egg allergy. Egg allergy may be confirmed by a consistent medical history of adverse reactions to eggs and egg-containing foods, plus skin and/or blood testing for immunoglobulin E antibodies to egg proteins. 2 If there is not previous exposure to eggs but suspicions of egg allergies exist due to prior allergy testing, give RIV if available, or refer to a health care provider with expertise in management of allergic conditions. 3 Vaccines should only be administered in settings where staff are familiar with and have appropriate equipment for response to anaphylactic reactions. Source: Centers for Disease Control and Prevention: Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 Influenza Season, found at www.cdc.gov/mmwr/preview/mmwrhtml/mm6430a3.htm Minnesota Dept. of Health – Immunization Program (9/15) Influenza vaccine dosing algorithm for children 6 months through 8 years old 2015 –16 Influenza Vaccination Season Did the child received 2* or more doses of trivalent or quadrivalent influenza vaccine before July 1, 2015? Yes No or Don’t Know 1 dose 2 doses at least 4 weeks apart *The two doses do not need to have been received during the same or consecutive seasons. Source: Centers for Disease Control and Prevention: Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 Influenza Season, found at www.cdc.gov/mmwr/preview/mmwrhtml/mm6430a3.htm Minnesota Dept. of Health – Immunization Program (9/15)