Inactivated Influenza Vaccine (IIV) Protocol Persons Age 9 Years and Older (Word)
advertisement

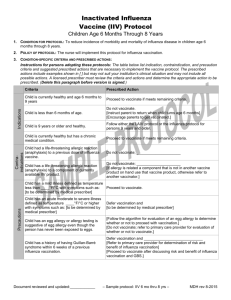
Inactivated Influenza Vaccine (IIV) Protocol Persons Age 9 Years or Older 1. CONDITION FOR PROTOCOL: To reduce incidence of morbidity and mortality of influenza disease. 2. POLICY OF PROTOCOL: The nurse will implement this protocol for seasonal influenza vaccination. Criteria Prescribed Action Person is currently healthy and age 9 years or older Proceed to vaccinate if meets remaining criteria. Person is less than age 9 years. Follow either the LAIV protocol or influenza protocol for persons 6 months through 8 year. Person is currently healthy but has a chronic medical condition. Proceed to vaccinate. Person is pregnant. Proceed to vaccinate Person had a life-threatening allergic reaction (anaphylaxis) to a previous dose of influenza vaccine. Do not vaccinate; _____________________ Contraindication s Indications 3. CONDITION-SPECIFIC CRITERIA AND PRESCRIBED ACTIONS: Instructions for persons adopting these protocols: The table below list indication, contraindication, and precaution criteria and suggested prescribed actions that are necessary to implement the vaccine protocol. The prescribed actions include examples shown in [ ] but may not suit your institution’s clinical situation and may not include all possible actions. A licensed prescriber must review the criteria and actions and determine the appropriate action to be prescribed. (Delete this paragraph before version is signed.) Do not vaccinate; _____________________ Person has a life-threatening allergic reaction [If allergy is related a component that is not in another vaccine (anaphylaxis) to a component of currently available product on hand use that vaccine product, otherwise refer to IIV product. another vaccinator.] Precautions Person has a mild illness defined as temperature less than ____°F/°C with symptoms such as: [to be Proceed to vaccinate. determined by medical prescriber] Person has an egg allergy. [Follow the attached egg allergy algorithm to determine whether or not to vaccinate. If the person cannot receive a vaccine with ovalbumin, give Recombinant Influenza Vaccine (RIV) or refer to another vaccinator that has RIV.] [Give RIV if available and person is 18 through 49 years old.] [Do not vaccinate; refer to primary care provider for further evaluation.] Person has an acute moderate to severe illness defined as temperature ____°F/°C or higher with symptoms such as: [to be determined by medical prescriber] Defer vaccination and _____________________ [to be determined by medical prescriber] Person has a history of having Guillan-Barré syndrome within 6 weeks of a previous influenza vaccination. [Refer to primary care provider for determination of risk and benefit of influenza vaccination] [Proceed to vaccinate after discussing risk and benefit of influenza vaccination and GBS.] Document reviewed and updated:____________ – Sample Protocol: IIV 9 years & older – MDH rev 08-2015 Sample – IIV Protocol - 9 Years & Older 4. PRESCRIPTION: GIVE ANY OF THE FOLLOWING PRODUCTS DEPENDING UPON WHICH IS AVAILABLE AND IF AGE APPROPRIATE. Product* Dose Route Age Indications Afluria, trivalent (IIV3) 0.5 mL IM 9 years and older† Fluarix, quadrivalent (IIV4) 0.5 mL IM 9 years and older† Flucelvax, cell culture inactivated influenza vaccine, trivalent (ccIIV3) 0.5 mL IM 18 years and older FluLaval, quadrivalent (IIV4) 0.5 mL IM 9 years and older† Fluvirin, trivalent (IIV3) 0.5 mL IM 9 years and older† Fluzone, trivalent (IIV3) or quadrivalent (IIV4) 0.5 mL IM 9 years and older† Fluzone ID, quadrivalent (IIV4) 0.1 mL ID 18 through 64 years of age Fluzone High-Dose, trivalent (IIV3) 0.5 mL IM 65 years and older Flublok, recombinant influenza vaccine, trivalent (RIV3) 0.5 mL IM 18 years and older *Use of product names are intended to help users delineate specific product indications and are not intended to be an endorsement of any particular product. † This vaccine is also licensed for younger age groups, see manufacturer package insert for details. 5. MEDICAL EMERGENCY OR ANAPHYLAXIS: [Depending on clinic staffing, include one of the two options below.] In the event of a medical emergency related to the administration of a vaccine. RN will apply protocols as described in ____________________________________________________________________________________________. In the event of an onset of symptoms of anaphylaxis including: o rash o itchiness of throat o difficulty breathing o bodily collapse o swollen tongue or throat LPN or unlicensed assistive personnel (MA) will immediately contact the RN in order to implement the ____________________________________________________________________________________________. 6. QUESTIONS OR CONCERNS: In the event of questions or concerns, call ____________________________at _____________________________. This protocol shall remain in effect for all patients of ______________________________until rescinded or until _____________________________________. Name of prescriber: _______________________________________________________________________________ Signature: ________________________________________________________________________________________ Date: ___________________________ Document reviewed and updated:____________ – Sample Protocol: IIV 9 years & older – MDH rev 08-2015 Algorithm for Evaluation of an Egg Allergy Preceding Influenza Vaccination, 2015 –16 Can the person eat lightly cooked egg (e.g., scrambled egg) without reactions? 1, 2 Yes Give vaccine per usual protocol. No After eating eggs or eggcontaining foods, does the person experience ONLY hives? Yes Give IIV if indicated and observe for reaction for at least 30 minutes after each dose.3 OR Give Recombinant Influenza Vaccine (RIV) if patient is 18 or older No Does the person experience other symptoms such as Cardiovascular changes (e.g., hypotension)? Respiratory distress (e.g., wheezing)? Gastrointestinal (e.g., nausea/vomiting)? Reaction requiring epinephrine? Give RIV if patient is 18 or older Yes OR IIV may be administered by a health care provider with advanced expertise in the management of allergic reactions (e.g., an allergist) Reaction requiring emergency medical attention? 1 Persons with egg allergy might tolerate egg in baked products (e.g., bread or cake). However, tolerance to egg-containing foods does not exclude the possibility of egg allergy. Egg allergy may be confirmed by a consistent medical history of adverse reactions to eggs and egg-containing foods, plus skin and/or blood testing for immunoglobulin E antibodies to egg proteins. 2 If there is not previous exposure to eggs but suspicions of egg allergies exist due to prior allergy testing, give RIV if available, or refer to a health care provider with expertise in management of allergic conditions. 3 Vaccines should only be administered in settings where staff are familiar with and have appropriate equipment for response to anaphylactic reactions. Source: Centers for Disease Control and Prevention: Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 Influenza Season, found at www.cdc.gov/mmwr/preview/mmwrhtml/mm6430a3.htm Minnesota Dept. of Health – Immunization Program (9/15)