Frontal Polymerization Laboratory Procedure
advertisement

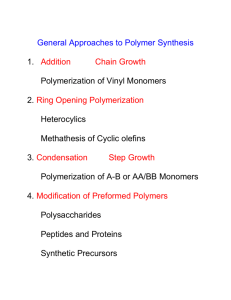
Thermal Frontal Polymerization John A. Pojman Fall 2009 Introduction Thermal frontal polymerization is a localized reaction zone that then propagates through the reactants by the coupling of thermal diffusion and the Arrhenius kinetics of an exothermic polymerization. The simplest example is a front propagating along the length of a glass tube. For more information http://pojman.com/FP.html 1) Pojman, J. A.; Ilyashenko, V. M.; Khan, A. M. "Free-Radical Frontal Polymerization: SelfPropagating Thermal Reaction Waves," J. Chem. Soc. Faraday Trans. 1996, 92, 2825-2837. 2) Pojman, J. A.; Varisli, B.; Perryman, A.; Edwards, C.; Hoyle, C. "Frontal Polymerization with Thiol-Ene Systems,"Macromolecules 2004, 37, 691-693. 3) McFarland, B.; Popwell, S.; Pojman, J. A. "Free-Radical Frontal Polymerization with a Microencapsulated Initiator," Macromolecules 2004, 37, 6670 - 6672. 4) Nason, C.; Roper, T.; Hoyle, C.; Pojman, J. A. "UV-Induced Frontal Polymerization of Multifunctional (Meth)Acrylates," Macromolecules 2005, 38, 5506-5512. Experimental Procedure All reactions should be performed in a hood with the glass shield down!! The reactions become very hot (over 200 ˚C). Hot peroxide is a very strong oxidizing agent. Wear poly(acrylonitrile) gloves when handling reagents You will need Polygloss 90 The peroxide initiator is 1,1-Bis(tert-butylperoxy)-3,3,5-trimethylcyclohexane (Luperox® 231) You use three monomers: 1,4-Butanediol diacrylate (BDDA) Butanediol dimethacrylate(BDMA) Trimethylol propane triacrylate (TMPTA) Dissolve 1 mL of Luperox 231 in 20 mL of each monomer. Mix in 8 g of Polygloss 90 (kaolin clay) until the material has a putty-like consistency. Apply to a wooden surface in two different sections with different thicknesses. Make one section 1 cm thick and the other 0.5 cm thick. Mark along the board 1 cm distances. (1) Start the front using the soldering iron. Once the front starts propagating, close the hood and monitor the front. Note the time the front reaches each cm mark. Have one person watch the front and another record the times. Time (min.) Position (cm) 0 0 ??? 1 ??? 2 Etc. Analysis 1) Plot the Position vs. time data, preferably all on one graph. 2) Calculate the front velocity for each experiment from the slope of the line fit to the position vs .time data. 3) Draw the structures of all the reagents. 4) Propose one or more hypotheses about what the observed front velocities and the structure of the monomers and how you could test each hypothesis. Specifically, consider this question: Why should a triacrylate front propagate faster than a diacrylate one? Why a diacrylate faster than a dimethacrylate?