Lecture Notes - Cloudfront.net
advertisement

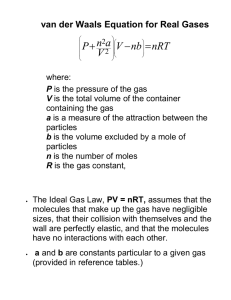
Lack of Intermolecular Forces in Gases(g) What’s the best way to describe gases at the chemical level? Q: Answer 1. Think of gases as consisting of individual molecules like O2, N2, CH4 ect…(see O2, N2, CH4, & SO2 overhead) 2. These individual molecules are in “constant random motion with no pattern.” They are “fluid” 3. They can and will collide with each other & the walls of their containter(pressure), but because they are moving so fast, they do not attract or repel each other. 4. The forces between them are “negligible” Q: How is this explanation at the chemical level observed in the world around me? A: Think hard about these examples(see burning match, see ballon.)