Final Review
advertisement

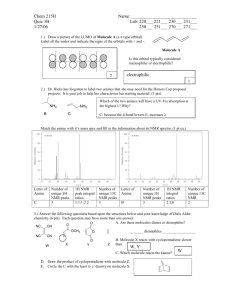
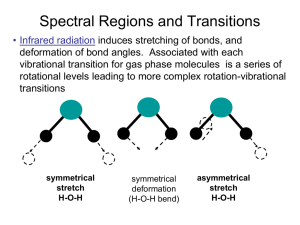
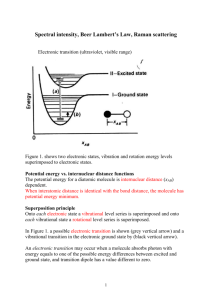
Final Review The final exam is on Tuesday May 7, at 12:30 in the regular Room. It will consist of two parts, a multiple choice section which will cover the entire semester and the second part, which will be over material we covered since the last exam. Circular Dichroism, NMR , Raman, IR etc. For the 1st part look over previous exams, quizzes, and exam reviews, and the review below. If you have time, go through old homework and work homework problems from the new material. The absorption/emission spectroscopy that we talked about in class were: IR absorption Microwave absorption UV-Vis Absorption Circular Dichroism MR We talked about the selection rules associated with IR, must have a changing dipole associated with the vibrational mode and for strictly harmonic oscillators v = +/- 1. How many normal modes are present for a particular molecule, 3N-5 or 3N-6. What is the fundamental transition, what is an overtone. The Morse potential allows one to include the anharmonicity of the vibrations. It becomes important at high values of the vibrational quantum number v. What is the expression for the vibrational energy when anharmonicity is included? What about rotational spectra selection rules? J = + /-1 or 0. The ideal model is the Rigid Rotor. Deviation from ideality comes from the centrifugal distortion constant. This is only important at high J values. What does the expression for the rotational energy like if this is included. If both the vibrational and rotational energy levels are changing, then the spectrum has some extra structure on it. There can be a P,Q, and R branch. What does each of these represent? What is an overtone, how about a combination band? Another type of interaction of light with matter is scattering. We talked about Rayleigh Scattering, Raman Scattering (both Stokes and anti-Stokes Raman Scattering) and we also talked about the selection rule that the polarizability of the molecule must change as a result of the vibration for the vibrational mode to be Raman active. Both IR and Raman Spectroscopy probe the vibrational modes of the molecule. How many vibrational modes are there for a given molecule? It depends on if they are linear or nonlinear, why? The birefringence in a molecule and chirality lead to optical rotation and circular dichroism being useful as a probe for molecules with chiral structures. Thus molecules can be characterized by polarized light. You should be able to explain how these techniques can be used and what the difference is between the two. Is the circular dichroism spectrum the same for the nucleoside or amino acid as it is for the polynucleotide or the protein? What structural affects contribute. Another topic is NMR which stands for ? This spectroscopy monitors changes in the nuclear spin state. Nuclei can have both integral and half integral spin states. In the presence of a magnetic field the spin states of a nucleus are no longer degenerate and the energy separation between the states depends on the strength of the field and the gyromagnetic ratio of the nucleus. That is E = h/2 B0 = hL where L is the frequency of the transition in the imposed magnetic field and is called the Larmor frequency. The Larmor frequency is 500MHz for a proton in a field Bo = 11.7 T. Given a table of , you should be able to give the Larmor frequency for other spin ½ nuclei as well. What are some of the common spin ½ nuclei. If an NMR instrument is a 300MHz instrument, what would the value of its magnetic field strength be. Why is NMR not considered to be a very sensitive technique in the sense that it can measure concentration only at the % level? In NMR two important features that alter the transition frequency band are chemical splitting and spin spin coupling. You should be able to explain each of these? Also given a spectrum and a molecule you should be able to identify which peaks are associated with a given NMR band. What is the spin-lattice relaxation time constant T1 and what is the other relaxation constant that is related to the bandwidth of the NMR peaks. For the two dimensional NMR techniques, what is the difference between COSY and NOESY. What is the Nuclear Overhauser Effect. What is it dependence on distance. About how far away will neighbors make an effect? What are Boltzmann statistics. How is the population of one energy related to the population of another energy level. How are these related to the temperature. In both IR and UV-Vis energy diagrams showing absorption and emission, we generally showed absorptions that started from the ground state of the molecule. Why should they start from the ground state, why not an excited state? We'll this is based on statistical Thermodynamics and the Boltzmann distribution (see Chp 11). Basically the relative populations of two states is give by n1/n0 = g exp(-(E1-E0)/kT). kT is about 200 cm-1 at room temperature, and a typical IR transition occurs at 2350 cm-1 (assymmetric stretch in CO2). Thus there is a small number in the excited state